Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging
- PMID: 19435319
- PMCID: PMC3345198
- DOI: 10.1021/ar800223c
Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging
Abstract
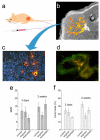
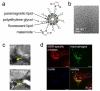
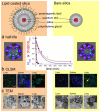
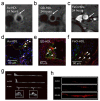
Modern medicine has greatly benefited from recent dramatic improvements in imaging techniques. The observation of physiological events through interactions manipulated at the molecular level offers unique insight into the function (and dysfunction) of the living organism. The tremendous advances in the development of nanoparticulate molecular imaging agents over the past decade have made it possible to noninvasively image the specificity, pharmacokinetic profiles, biodistribution, and therapeutic efficacy of many novel compounds. Several types of nanoparticles have demonstrated utility for biomedical purposes, including inorganic nanocrystals, such as iron oxide, gold, and quantum dots. Moreover, natural nanoparticles, such as viruses, lipoproteins, or apoferritin, as well as hybrid nanostructures composed of inorganic and natural nanoparticles, have been applied broadly. However, among the most investigated nanoparticle platforms for biomedical purposes are lipidic aggregates, such as liposomal nanoparticles, micelles, and microemulsions. Their relative ease of preparation and functionalization, as well as the ready synthetic ability to combine multiple amphiphilic moieties, are the most important reasons for their popularity. Lipid-based nanoparticle platforms allow the inclusion of a variety of imaging agents, ranging from fluorescent molecules to chelated metals and nanocrystals. In recent years, we have created a variety of multifunctional lipid-based nanoparticles for molecular imaging; many are capable of being used with more than one imaging technique (that is, with multimodal imaging ability). These nanoparticles differ in size, morphology, and specificity for biological markers. In this Account, we discuss the development and characterization of five different particles: liposomes, micelles, nanocrystal micelles, lipid-coated silica, and nanocrystal high-density lipoprotein (HDL). We also demonstrate their application for multimodal molecular imaging, with the main focus on magnetic resonance imaging (MRI), optical techniques, and transmission electron microscopy (TEM). The functionalization of the nanoparticles and the modulation of their pharmacokinetics are discussed. Their application for molecular imaging of key processes in cancer and cardiovascular disease are shown. Finally, we discuss a recent development in which the endogenous nanoparticle HDL was modified to carry different diagnostically active nanocrystal cores to enable multimodal imaging of macrophages in experimental atherosclerosis. The multimodal characteristics of the different contrast agent platforms have proven to be extremely valuable for validation purposes and for understanding mechanisms of particle-target interaction at different levels, ranging from the entire organism down to cellular organelles.
Figures







Similar articles
-
Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging.NMR Biomed. 2006 Feb;19(1):142-64. doi: 10.1002/nbm.1011. NMR Biomed. 2006. PMID: 16450332 Review.
-
Paramagnetic lipid-coated silica nanoparticles with a fluorescent quantum dot core: a new contrast agent platform for multimodality imaging.Bioconjug Chem. 2008 Dec;19(12):2471-9. doi: 10.1021/bc800368x. Bioconjug Chem. 2008. PMID: 19035793 Free PMC article.
-
High-Density Lipoprotein Nanobiologics for Precision Medicine.Acc Chem Res. 2018 Jan 16;51(1):127-137. doi: 10.1021/acs.accounts.7b00339. Epub 2017 Dec 27. Acc Chem Res. 2018. PMID: 29281244 Free PMC article. Review.
-
Nanocrystal core high-density lipoproteins: a multimodality contrast agent platform.Nano Lett. 2008 Nov;8(11):3715-23. doi: 10.1021/nl801958b. Epub 2008 Oct 22. Nano Lett. 2008. PMID: 18939808 Free PMC article.
-
Silica-shelled single quantum dot micelles as imaging probes with dual or multimodality.Anal Chem. 2006 Aug 15;78(16):5925-32. doi: 10.1021/ac060412b. Anal Chem. 2006. PMID: 16906742
Cited by
-
Augmenting drug-carrier compatibility improves tumour nanotherapy efficacy.Nat Commun. 2016 Apr 13;7:11221. doi: 10.1038/ncomms11221. Nat Commun. 2016. PMID: 27071376 Free PMC article.
-
A non-invasive nanoparticles for multimodal imaging of ischemic myocardium in rats.J Nanobiotechnology. 2021 Mar 22;19(1):82. doi: 10.1186/s12951-021-00822-7. J Nanobiotechnology. 2021. PMID: 33752679 Free PMC article.
-
Nanomaterial-based contrast agents.Nat Rev Methods Primers. 2023;3:30. doi: 10.1038/s43586-023-00211-4. Epub 2023 Apr 13. Nat Rev Methods Primers. 2023. PMID: 38130699 Free PMC article.
-
Gold nanoshelled liquid perfluorocarbon magnetic nanocapsules: a nanotheranostic platform for bimodal ultrasound/magnetic resonance imaging guided photothermal tumor ablation.Theranostics. 2013 Dec 1;4(1):12-23. doi: 10.7150/thno.7275. eCollection 2013. Theranostics. 2013. PMID: 24396512 Free PMC article.
-
Nanoparticles for biomedical imaging: fundamentals of clinical translation.Mol Imaging. 2010 Dec;9(6):291-310. Mol Imaging. 2010. PMID: 21084027 Free PMC article. Review.
References
-
- Mulder WJ, Cormode DP, Hak S, Lobatto ME, Silvera S, Fayad ZA. Multimodality Nanotracers for Cardiovascular Applications. Nat. Clin. Pract. Cardiovasc. Med. 2008;5(Suppl 2):S103–S111. - PubMed
-
- Sanhai WR, Sakamoto JH, Canady R, Ferrari M. Seven Challenges for Nanomedicine. Nat. Nanotechnol. 2008;3:242–244. - PubMed
-
- Weissleder R, Mahmood U. Molecular Imaging. Radiology. 2001;219:316–333. - PubMed
-
- Mulder WJ, Strijkers GJ, van Tilborg GA, Griffioen AW, Nicolay K. Lipid-Based Nanoparticles for Contrast-Enhanced MRI and Molecular Imaging. NMR Biomed. 2006;19:142–164. - PubMed
-
- Liu Y, Miyoshi H, Nakamura M. Nanomedicine for Drug Delivery and Imaging: a Promising Avenue for Cancer Therapy and Diagnosis Using Targeted Functional Nanoparticles. Int. J. Cancer. 2007;120:2527–2537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

