Human papillomavirus infection and cervical cytology in HIV-infected and HIV-uninfected Rwandan women
- PMID: 19435429
- PMCID: PMC2814215
- DOI: 10.1086/599123
Human papillomavirus infection and cervical cytology in HIV-infected and HIV-uninfected Rwandan women
Abstract
Background: Data on human papillomavirus (HPV) prevalence are essential for developing cost-effective cervical cancer prevention programs.
Methods: In 2005, 710 human immunodeficiency virus (HIV)-positive and 226 HIV-negative Rwandan women enrolled in an observational prospective cohort study. Sociodemographic data, CD4+ cell counts, and cervical specimens were obtained. Cervicovaginal lavage specimens were collected from each woman and tested for >40 HPV types by a polymerase chain reaction assay; HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 were considered primary carcinogenic HPV types.
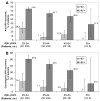
Results: The prevalence of HPV was higher in HIV-positive women than in HIV-negative women in all age groups. Among HIV-infected women, 69% were positive for >or=1 HPV type, 46% for a carcinogenic HPV type, and 10% for HPV-16. HPV prevalence peaked at 75% in the HIV-positive women aged 25-34 years and then declined with age to 37.5% in those >or=55 years old (Ptrend<.001). A significant trend of higher prevalence of HPV and carcinogenic HPV with lower CD4+ cell counts and increasing cytologic severity was seen among HIV-positive women.
Conclusions: We found a higher prevalence of HPV infection in HIV-positive than in HIV-negative Rwandan women, and the prevalence of HPV and carcinogenic HPV infection decreased with age.
Conflict of interest statement
Potential conflicts of interest: none reported.
Figures
References
-
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. - PubMed
-
- Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–55. - PubMed
-
- Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. - PubMed
-
- Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multi-centre phase II efficacy trial. Lancet Oncol. 2005;6:271–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

