Reporting of sample size calculation in randomised controlled trials: review
- PMID: 19435763
- PMCID: PMC2680945
- DOI: 10.1136/bmj.b1732
Reporting of sample size calculation in randomised controlled trials: review
Abstract
Objectives: To assess quality of reporting of sample size calculation, ascertain accuracy of calculations, and determine the relevance of assumptions made when calculating sample size in randomised controlled trials.
Design: Review.
Data sources: We searched MEDLINE for all primary reports of two arm parallel group randomised controlled trials of superiority with a single primary outcome published in six high impact factor general medical journals between 1 January 2005 and 31 December 2006. All extra material related to design of trials (other articles, online material, online trial registration) was systematically assessed. Data extracted by use of a standardised form included parameters required for sample size calculation and corresponding data reported in results sections of articles. We checked completeness of reporting of the sample size calculation, systematically replicated the sample size calculation to assess its accuracy, then quantified discrepancies between a priori hypothesised parameters necessary for calculation and a posteriori estimates.
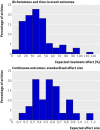
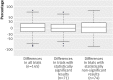
Results: Of the 215 selected articles, 10 (5%) did not report any sample size calculation and 92 (43%) did not report all the required parameters. The difference between the sample size reported in the article and the replicated sample size calculation was greater than 10% in 47 (30%) of the 157 reports that gave enough data to recalculate the sample size. The difference between the assumptions for the control group and the observed data was greater than 30% in 31% (n=45) of articles and greater than 50% in 17% (n=24). Only 73 trials (34%) reported all data required to calculate the sample size, had an accurate calculation, and used accurate assumptions for the control group.
Conclusions: Sample size calculation is still inadequately reported, often erroneous, and based on assumptions that are frequently inaccurate. Such a situation raises questions about how sample size is calculated in randomised controlled trials.
Conflict of interest statement
Competing interests: None declared.
Figures



References
-
- ICH Harmonised Tripartite Guideline. Statistical principles for clinical trials. International Conference on Harmonisation E9 Expert Working Group. Stat Med 1999;18:1905-42. - PubMed
-
- Moher D, Dulberg CS, Wells GA. Statistical power, sample size, and their reporting in randomized controlled trials. JAMA 1994;272:122-4. - PubMed
-
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001;134:663-94. - PubMed
-
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4. - PubMed
-
- Machin D, Campbell M, Fayers P, Pinol A. Sample size tables for clinical studies. 2nd ed. Oxford: Blackwell Science, 1997.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
