Compartmentalization of immune responses in human tuberculosis: few CD8+ effector T cells but elevated levels of FoxP3+ regulatory t cells in the granulomatous lesions
- PMID: 19435796
- PMCID: PMC2684186
- DOI: 10.2353/ajpath.2009.080941
Compartmentalization of immune responses in human tuberculosis: few CD8+ effector T cells but elevated levels of FoxP3+ regulatory t cells in the granulomatous lesions
Abstract
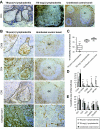
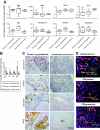
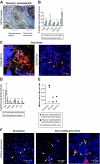
Immune responses were assessed at the single-cell level in lymph nodes from children with tuberculous lymphadenitis. Tuberculosis infection was associated with tissue remodeling of lymph nodes as well as altered cellular composition. Granulomas were significantly enriched with CD68+ macrophages expressing the M. tuberculosis complex-specific protein antigen MPT64 and inducible nitric oxide synthase. There was a significant increase in CD8+ cytolytic T cells surrounding the granuloma; however, CD8+ T cells expressed low levels of the cytolytic and antimicrobial effector molecules perforin and granulysin in the granulomatous lesions. Quantitative real-time mRNA analysis revealed that interferon-gamma, tumor necrosis factor-alpha, and interleukin-17 were not up-regulated in infected lymph nodes, but there was a significant induction of both transforming growth factor-beta and interleukin-13. In addition, granulomas contained an increased number of CD4+FoxP3+ T cells co-expressing the immunoregulatory cytotoxic T-lymphocyte antigen-4 and glucocorticoid-induced tumor necrosis factor receptor molecules. Low numbers of CD8+ T cells in the lesions correlated with high levels of transforming growth factor-beta and FoxP3+ regulatory T cells, suggesting active immunosuppression at the local infection site. Compartmentalization and skewing of the immune response toward a regulatory phenotype may result in an uncoordinated effector T-cell response that reduces granule-mediated killing of M. tuberculosis-infected cells and subsequent disease control.
Figures

References
-
- Fenhalls G, Stevens-Muller L, Warren R, Carroll N, Bezuidenhout J, Van Helden P, Bardin P. Localisation of mycobacterial DNA and mRNA in human tuberculous granulomas. J Microbiol Methods. 2002;51:197–208. - PubMed
-
- Ulrichs T, Kosmiadi GA, Trusov V, Jorg S, Pradl L, Titukhina M, Mishenko V, Gushina N, Kaufmann SH. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol. 2004;204:217–228. - PubMed
-
- Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–1949. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

