A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells
- PMID: 19435801
- PMCID: PMC2684836
- DOI: 10.1242/jcs.046870
A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells
Erratum in
- J Cell Sci. 2009 Aug 15;122(Pt 16):3005
Abstract
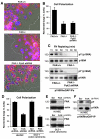
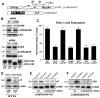
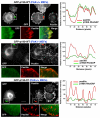
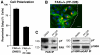
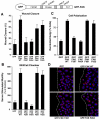
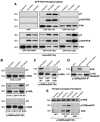
Directional motility is a complex process requiring the spatiotemporal integration of signals that regulate cytoskeletal changes, and the establishment of an anteroposterior or polarized cell axis. Focal adhesion kinase (FAK) promotes cell migration, but a molecular role for FAK in promoting cell polarity remains undefined. Here, using wound healing and Golgi-reorientation analyses, we show that fibroblast, endothelial and carcinoma polarity during cell migration requires FAK and is associated with a complex between FAK, p120RasGAP and p190RhoGAP (p190A), leading to p190A tyrosine phosphorylation. Fibronectin-integrin-mediated FAK activation and phosphorylation promote SH2-mediated binding of p120RasGAP to FAK and FAK-mediated p190A tyrosine phosphorylation. The association of p120RasGAP with FAK facilitates the formation of a FAK-p120RasGAP-p190A complex targeted to leading-edge focal adhesions by FAK. Knockdown of p120RasGAP, mutation of FAK Y397 or inhibition of FAK activity prevent the association of FAK with p190A and subsequent tyrosine phosphorylation of p190A, and result in the loss of cell polarity. Because reconstitution of FAK-null fibroblasts with FAK or a Pyk2-FAK chimera restore the normal decrease in RhoA GTP binding upon cell spreading on fibronectin, our studies support a model whereby FAK activity facilitates the recruitment and stabilization of a p120RasGAP-p190A complex at leading-edge focal adhesions connected to the transient inhibition of RhoA activity and the regulation of cell polarity.
Figures




References
-
- Arthur, W. T., Petch, L. A. and Burridge, K. (2000). Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10, 719-722. - PubMed
-
- Beardsley, A., Fang, K., Mertz, H., Castranova, V., Friend, S. and Liu, J. (2005). Loss of caveolin-1 polarity impedes endothelial cell polarization and directional movement. J. Biol. Chem. 280, 3541-3547. - PubMed
-
- Bernards, A. and Settleman, J. (2004). GAP control: regulating the regulators of small GTPases. Trends Cell Biol. 14, 377-385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

