Role of the protein tyrosine kinase Syk in regulating cell-cell adhesion and motility in breast cancer cells
- PMID: 19435818
- PMCID: PMC2788757
- DOI: 10.1158/1541-7786.MCR-08-0371
Role of the protein tyrosine kinase Syk in regulating cell-cell adhesion and motility in breast cancer cells
Abstract
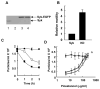
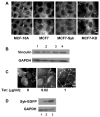
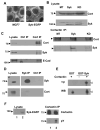
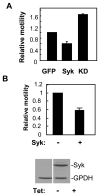
The expression of the Syk protein tyrosine kinase in breast cancer cells is inversely correlated with invasive growth and metastasis. The expression of Syk inhibits cell motility while supporting the formation of cell clusters by enhancing cell-cell contacts and promoting the redistribution of the adhesion proteins cortactin and vinculin to these contacts. Syk associates physically with cortactin and catalyzes its phosphorylation on tyrosine. The clustering of integrins leads to the phosphorylation of Syk and of numerous cellular proteins in a manner dependent on the activity of the kinase and on the presence of tyrosine 342 located in the linker B region. The ability of Syk to participate in integrin-mediated protein tyrosine phosphorylation correlates well with its ability to inhibit cell motility.
Figures



References
-
- Turner M, Schweighoffer E, Colucci F, Di Santo JP, Tybulewicz VLJ. Tyrosine kinase SYK: essential functions for immunoreceptor signalling. Immunol Today. 2000;21:148–54. - PubMed
-
- Yanagi S, Inatome R, Takano T, Yamamura H. Syk expression and novel function in a wide variety of tissues. Biochem Biophys Res Commun. 2001;288:495–8. - PubMed
-
- Coopman PJ, Do MT, Barth M, et al. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature. 2000;406:742–7. - PubMed
-
- Yuan Y, Mendez R, Sahin A, Dai JL. Hypermethylation leads to silencing of the SYK gene in human breast cancer. Cancer Res. 2001;61:5558–61. - PubMed
-
- Moroni M, Soldatenkov V, Zhang L, et al. Progressive loss of Syk and abnormal proliferation in breast cancer cells. Cancer Res. 2004;64:7346–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

