Diabetes increases brain damage caused by severe hypoglycemia
- PMID: 19435850
- PMCID: PMC2711670
- DOI: 10.1152/ajpendo.91041.2008
Diabetes increases brain damage caused by severe hypoglycemia
Abstract
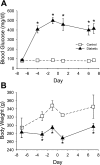
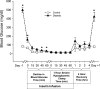
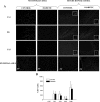
Insulin-induced severe hypoglycemia causes brain damage. The hypothesis to be tested was that diabetes portends to more extensive brain tissue damage following an episode of severe hypoglycemia. Nine-week-old male streptozotocin-diabetic (DIAB; n = 10) or vehicle-injected control (CONT; n = 7) Sprague-Dawley rats were subjected to hyperinsulinemic (0.2 U.kg(-1).min(-1)) severe hypoglycemic (10-15 mg/dl) clamps while awake and unrestrained. Groups were precisely matched for depth and duration (1 h) of severe hypoglycemia (CONT 11 +/- 0.5 and DIAB 12 +/- 0.2 mg/dl, P = not significant). During severe hypoglycemia, an equal number of episodes of seizure-like activity were noted in both groups. One week later, histological analysis demonstrated extensive neuronal damage in regions of the hippocampus, especially in the dentate gyrus and CA1 regions and less so in the CA3 region (P < 0.05), although total hippocampal damage was not different between groups. However, in the cortex, DIAB rats had significantly (2.3-fold) more dead neurons than CONT rats (P < 0.05). There was a strong correlation between neuronal damage and the occurrence of seizure-like activity (r(2) > 0.9). Separate studies conducted in groups of diabetic (n = 5) and nondiabetic (n = 5) rats not exposed to severe hypoglycemia showed no brain damage. In summary, under the conditions studied, severe hypoglycemia causes brain damage in the cortex and regions within the hippocampus, and the extent of damage is closely correlated to the presence of seizure-like activity in nonanesthetized rats. It is concluded that, in response to insulin-induced severe hypoglycemia, diabetes uniquely increases the vulnerability of specific brain areas to neuronal damage.
Figures



References
-
- Alkire MT, Pomfrett CJ, Haier RJ, Gianzero MV, Chan CM, Jacobsen BP, Fallon JH. Functional brain imaging during anesthesia in humans: effects of halothane on global and regional cerebral glucose metabolism. Anesthesiology 90: 701–709, 1999. - PubMed
-
- Auer RN Hypoglycemic brain damage. Metab Brain Dis 19: 169–175, 2004. - PubMed
-
- Auer RN, Hugh J, Cosgrove E, Curry B. Neuropathologic findings in three cases of profound hypoglycemia. Clin Neuropathol 8: 63–68, 1989. - PubMed
-
- Austin EJ, Deary IJ. Effects of repeated hypoglycemia on cognitive function: a psychometrically validated reanalysis of the Diabetes Control and Complications Trial data. Diabetes Care 22: 1273–1277, 1999. - PubMed
-
- Baird TA, Parsons MW, Phanh T, Butcher KS, Desmond PM, Tress BM, Colman PG, Chambers BR, Davis SM. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke 34: 2208–2214, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

