The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells
- PMID: 19435897
- PMCID: PMC2727355
- DOI: 10.1158/0008-5472.CAN-08-4859
The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells
Abstract
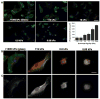
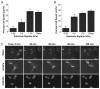
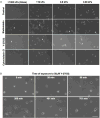
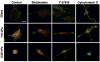
Glioblastoma multiforme (GBM) is a malignant astrocytoma of the central nervous system associated with a median survival time of 15 months, even with aggressive therapy. This rapid progression is due in part to diffuse infiltration of single tumor cells into the brain parenchyma, which is thought to involve aberrant interactions between tumor cells and the extracellular matrix (ECM). Here, we test the hypothesis that mechanical cues from the ECM contribute to key tumor cell properties relevant to invasion. We cultured a series of glioma cell lines (U373-MG, U87-MG, U251-MG, SNB19, C6) on fibronectin-coated polymeric ECM substrates of defined mechanical rigidity and investigated the role of ECM rigidity in regulating tumor cell structure, migration, and proliferation. On highly rigid ECMs, tumor cells spread extensively, form prominent stress fibers and mature focal adhesions, and migrate rapidly. As ECM rigidity is lowered to values comparable with normal brain tissue, tumor cells appear rounded and fail to productively migrate. Remarkably, cell proliferation is also strongly regulated by ECM rigidity, with cells dividing much more rapidly on rigid than on compliant ECMs. Pharmacologic inhibition of nonmuscle myosin II-based contractility blunts this rigidity-sensitivity and rescues cell motility on highly compliant substrates. Collectively, our results provide support for a novel model in which ECM rigidity provides a transformative, microenvironmental cue that acts through actomyosin contractility to regulate the invasive properties of GBM tumor cells.
Conflict of interest statement
Figures
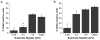
References
-
- Manning TJ, Jr, Parker JC, Sontheimer H. Role of lysophosphatidic acid and rho in glioma cell motility. Cell Motil Cytoskeleton. 2000;45:185–99. - PubMed
-
- Salhia B, Rutten F, Nakada M, et al. Inhibition of Rho-kinase affects astrocytoma morphology, motility, and invasion through activation of Rac1. Cancer Res. 2005;65:8792–800. - PubMed
-
- Mahesparan R, Tysnes BB, Read TA, Enger PO, Bjerkvig R, Lund-Johansen M. Extracellular matrix-induced cell migration from glioblastoma biopsy specimens in vitro. Acta Neuropathol. 1999;97:231–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

