Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer
- PMID: 19435900
- PMCID: PMC2699841
- DOI: 10.1158/0008-5472.CAN-08-4698
Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer
Abstract
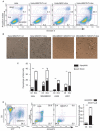
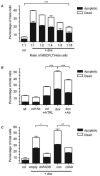
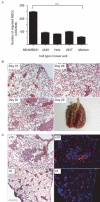
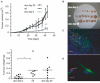
Cancer is a leading cause of mortality throughout the world and new treatments are urgently needed. Recent studies suggest that bone marrow-derived mesenchymal stem cells (MSC) home to and incorporate within tumor tissue. We hypothesized that MSCs engineered to produce and deliver tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a transmembrane protein that causes selective apoptosis of tumor cells, would home to and kill cancer cells in a lung metastatic cancer model. Human MSCs were transduced with TRAIL and the IRES-eGFP reporter gene under the control of a tetracycline promoter using a lentiviral vector. Transduced and activated MSCs caused lung (A549), breast (MDAMB231), squamous (H357), and cervical (Hela) cancer cell apoptosis and death in coculture experiments. Subcutaneous xenograft experiments confirmed that directly delivered TRAIL-expressing MSCs were able to significantly reduce tumor growth [0.12 cm(3) (0.04-0.21) versus 0.66 cm(3) (0.21-1.11); P < 0.001]. We then found, using a pulmonary metastasis model, systemically delivered MSCs localized to lung metastases and the controlled local delivery of TRAIL completely cleared the metastatic disease in 38% of mice compared with 0% of controls (P < 0.05). This is the first study to show a significant reduction in metastatic tumor burden with frequent eradication of metastases using inducible TRAIL-expressing MSCs. This has a wide potential therapeutic role, which includes the treatment of both primary tumors and their metastases, possibly as an adjuvant therapy in clearing micrometastatic disease following primary tumor resection.
Figures


Similar articles
-
Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour.Int J Mol Sci. 2018 Jul 27;19(8):2188. doi: 10.3390/ijms19082188. Int J Mol Sci. 2018. PMID: 30060445 Free PMC article. Review.
-
Systemic but not topical TRAIL-expressing mesenchymal stem cells reduce tumour growth in malignant mesothelioma.Thorax. 2014 Jul;69(7):638-47. doi: 10.1136/thoraxjnl-2013-204110. Epub 2014 Feb 24. Thorax. 2014. PMID: 24567297 Free PMC article.
-
Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells.Neurosurgery. 2009 Sep;65(3):610-24; discussion 624. doi: 10.1227/01.NEU.0000350227.61132.A7. Neurosurgery. 2009. PMID: 19687708
-
TRAIL-expressing gingival-derived mesenchymal stem cells inhibit tumorigenesis of tongue squamous cell carcinoma.J Dent Res. 2015 Jan;94(1):219-28. doi: 10.1177/0022034514557815. Epub 2014 Nov 12. J Dent Res. 2015. PMID: 25391621
-
Mesenchymal stem cells as vectors for lung cancer therapy.Respiration. 2013;85(6):443-51. doi: 10.1159/000351284. Epub 2013 May 23. Respiration. 2013. PMID: 23711430 Free PMC article. Review.
Cited by
-
Emerging role of mesenchymal stromal cells in gynecologic cancer therapy.Stem Cell Res Ther. 2023 Dec 5;14(1):347. doi: 10.1186/s13287-023-03585-0. Stem Cell Res Ther. 2023. PMID: 38049868 Free PMC article. Review.
-
Great promise of tissue-resident adult stem/progenitor cells in transplantation and cancer therapies.Adv Exp Med Biol. 2012;741:171-86. doi: 10.1007/978-1-4614-2098-9_12. Adv Exp Med Biol. 2012. PMID: 22457110 Free PMC article. Review.
-
Migration of mesenchymal stem cells to tumor xenograft models and in vitro drug delivery by doxorubicin.Int J Med Sci. 2018 Jun 22;15(10):1051-1061. doi: 10.7150/ijms.25760. eCollection 2018. Int J Med Sci. 2018. PMID: 30013447 Free PMC article.
-
Mesenchymal Stem Cell Expressing TRAIL as Targeted Therapy against Sensitised Tumour.Int J Mol Sci. 2018 Jul 27;19(8):2188. doi: 10.3390/ijms19082188. Int J Mol Sci. 2018. PMID: 30060445 Free PMC article. Review.
-
Stem Cell Therapy for Thyroid Diseases: Progress and Challenges.Curr Ther Res Clin Exp. 2022 Mar 5;96:100665. doi: 10.1016/j.curtheres.2022.100665. eCollection 2022. Curr Ther Res Clin Exp. 2022. PMID: 35371349 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Menon LG, Picinich S, Koneru R, et al. Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells. 2007;25:520–8. - PubMed
-
- Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. - PubMed
-
- Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–603. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

