New definition for the partial remission period in children and adolescents with type 1 diabetes
- PMID: 19435955
- PMCID: PMC2713624
- DOI: 10.2337/dc08-1987
New definition for the partial remission period in children and adolescents with type 1 diabetes
Abstract
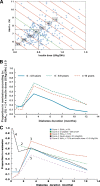
OBJECTIVE To find a simple definition of partial remission in type 1 diabetes that reflects both residual beta-cell function and efficacy of insulin treatment. RESEARCH DESIGN AND METHODS A total of 275 patients aged <16 years were followed from onset of type 1 diabetes. After 1, 6, and 12 months, stimulated C-peptide during a challenge was used as a measure of residual beta-cell function. RESULTS By multiple regression analysis, a negative association between stimulated C-peptide and A1C (regression coefficient -0.21, P < 0.001) and insulin dose (-0.94, P < 0.001) was shown. These results suggested the definition of an insulin dose-adjusted A1C (IDAA1C) as A1C (percent) + [4 x insulin dose (units per kilogram per 24 h)]. A calculated IDAA1C < or =9 corresponding to a predicted stimulated C-peptide >300 pmol/l was used to define partial remission. The IDAA1C < or =9 had a significantly higher agreement (P < 0.001) with residual beta-cell function than use of a definition of A1C < or =7.5%. Between 6 and 12 months after diagnosis, for IDAA1C < or =9 only 1 patient entered partial remission and 61 patients ended partial remission, for A1C < or =7.5% 15 patients entered partial remission and 53 ended, for a definition of insulin dose < or =0.5 units . kg(-1) . 24 h(-1) 5 patients entered partial remission and 66 ended, and for stimulated C-peptide (>300 pmol/l) 9 patients entered partial remission and 49 ended. IDAA1C at 6 months has good predictive power for stimulated C-peptide concentrations after both 6 and 12 months. CONCLUSIONS A new definition of partial remission is proposed, including both glycemic control and insulin dose. It reflects residual beta-cell function and has better stability compared with the conventional definitions.
Figures

References
-
- Buyukgebiz A, Cemeroglu AP, Bober E, Mohn A, Chiarelli F: Factors influencing remission phase in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2001; 14: 1585– 1596 - PubMed
-
- Hramiak IM, Dupre J, Finegood DT: Determinants of clinical remission in recent-onset IDDM. Diabetes Care 1993; 16: 125– 132 - PubMed
-
- Kordonouri O, Danne T, Enders I, Weber B: Does the long-term clinical course of type I diabetes mellitus differ in patients with prepubertal and pubertal onset? Results of the Berlin Retinopathy Study. Eur J Pediatr 1998; 157: 202– 207 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

