Alterations in cholesterol absorption/synthesis markers characterize Framingham offspring study participants with CHD
- PMID: 19436064
- PMCID: PMC2724787
- DOI: 10.1194/jlr.P900039-JLR200
Alterations in cholesterol absorption/synthesis markers characterize Framingham offspring study participants with CHD
Abstract
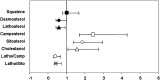
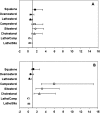
Data is limited on measures influencing cholesterol homeostasis in subjects at high risk of developing cardiovascular disease (CVD) relative to established risk factors. To address this, we quantified circulating indicators of cholesterol homeostasis (plasma phytosterols and cholesterol precursor concentrations as surrogate measures of cholesterol absorption and synthesis, respectively) in Framingham Offspring Study Cycle-6 participants diagnosed with established CVD and/or >or=50% carotid stenosis not taking lipid lowering medication (cases, N = 155) and matched controls (N = 414). Cases and controls had similar plasma LDL-cholesterol; HDL-cholesterol was significantly lower in males, while triglyceride concentrations were significantly higher in female cases relative to their respective controls. Cholesterol absorption markers were significantly higher (229 +/- 7 vs. 196 +/- 4, 169 +/- 6 vs. 149 +/- 3 and 144 +/- 5 vs. 135 +/- 3 for campesterol, sitosterol, and cholestanol, respectively), whereas cholesterol synthesis markers were significantly lower (116 +/- 4 vs. 138 +/- 3, 73 +/- 3 vs. 75 +/- 2 for lathosterol and desmosterol, respectively) in cases compared with controls, irrespective of sex. After controlling for standard risk factors, campesterol (2.47 [1.71-3.56]; P < 0.0001), sitosterol (1.86 [1.38-2.50]; P < 0.0001), cholestanol (1.57 [1.09-2.27]; P = 0.02), desmosterol (0.59 [0.42-0.84]; P = 0.003), and lathosterol (0.58 [0.43-0.77]; P = 0.0002) were significantly associated with CVD (odds ratio [95% confidence interval]). These data suggest that impaired cholesterol homeostasis, reflected by lower synthesis and higher absorption marker concentrations, are highly significant independent predictors of prevalent CVD in this study population.
Figures
References
-
- National Cholesterol Education Program (NCEP) Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults. 2002. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 106: 3143–3421. - PubMed
-
- Baigent C., Keech A., Kearney P. M., Blackwell L., Buck G., Pollicino C., Kirby A., Sourjina T., Peto R., Collins R., et al. for Cholesterol Treatment Trialists' (CTT) Collaborators. 2005. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 366: 1267–1278. - PubMed
-
- Heart Protection Study Collaborative Group. 2002. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 360: 23–33. - PubMed
-
- Long-term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. 1998. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N. Engl. J. Med. 339: 1349–1357. - PubMed
-
- Nakamura H., Arakawa K., Itakura H., Kitabatake A., Goto Y., Toyota T., Nakaya N., Nishimoto S., Muranaka M., Yamamoto A., et al. MEGA Study Group. 2006. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 368: 1155–1163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

