Prevention of hepatocarcinogenesis and increased susceptibility to acetaminophen-induced liver failure in transaldolase-deficient mice by N-acetylcysteine
- PMID: 19436114
- PMCID: PMC2689120
- DOI: 10.1172/JCI35722
Prevention of hepatocarcinogenesis and increased susceptibility to acetaminophen-induced liver failure in transaldolase-deficient mice by N-acetylcysteine
Abstract
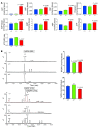
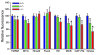
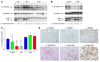
Although oxidative stress has been implicated in acute acetaminophen-induced liver failure and in chronic liver cirrhosis and hepatocellular carcinoma (HCC), no common underlying metabolic pathway has been identified. Recent case reports suggest a link between the pentose phosphate pathway (PPP) enzyme transaldolase (TAL; encoded by TALDO1) and liver failure in children. Here, we show that Taldo1-/- and Taldo1+/- mice spontaneously developed HCC, and Taldo1-/- mice had increased susceptibility to acetaminophen-induced liver failure. Oxidative stress in Taldo1-/- livers was characterized by the accumulation of sedoheptulose 7-phosphate, failure to recycle ribose 5-phosphate for the oxidative PPP, depleted NADPH and glutathione levels, and increased production of lipid hydroperoxides. Furthermore, we found evidence of hepatic mitochondrial dysfunction, as indicated by loss of transmembrane potential, diminished mitochondrial mass, and reduced ATP/ADP ratio. Reduced beta-catenin phosphorylation and enhanced c-Jun expression in Taldo1-/- livers reflected adaptation to oxidative stress. Taldo1-/- hepatocytes were resistant to CD95/Fas-mediated apoptosis in vitro and in vivo. Remarkably, lifelong administration of the potent antioxidant N-acetylcysteine (NAC) prevented acetaminophen-induced liver failure, restored Fas-dependent hepatocyte apoptosis, and blocked hepatocarcinogenesis in Taldo1-/- mice. These data reveal a protective role for the TAL-mediated branch of the PPP against hepatocarcinogenesis and identify NAC as a promising treatment for liver disease in TAL deficiency.
Figures





Comment in
-
TAL deficiency, all roads lead to oxidative stress?Hepatology. 2009 Sep;50(3):979-81. doi: 10.1002/hep.23227. Hepatology. 2009. PMID: 19714731 No abstract available.
Similar articles
-
mTOR-dependent loss of PON1 secretion and antiphospholipid autoantibody production underlie autoimmunity-mediated cirrhosis in transaldolase deficiency.J Autoimmun. 2023 Nov;140:103112. doi: 10.1016/j.jaut.2023.103112. Epub 2023 Sep 22. J Autoimmun. 2023. PMID: 37742509 Free PMC article.
-
Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase.Trends Mol Med. 2011 Jul;17(7):395-403. doi: 10.1016/j.molmed.2011.01.014. Epub 2011 Mar 2. Trends Mol Med. 2011. PMID: 21376665 Free PMC article. Review.
-
Transaldolase haploinsufficiency in subjects with acetaminophen-induced liver failure.J Inherit Metab Dis. 2020 May;43(3):496-506. doi: 10.1002/jimd.12197. Epub 2020 Jan 1. J Inherit Metab Dis. 2020. PMID: 31769880 Free PMC article.
-
Transaldolase deficiency influences the pentose phosphate pathway, mitochondrial homoeostasis and apoptosis signal processing.Biochem J. 2008 Oct 1;415(1):123-34. doi: 10.1042/BJ20080722. Biochem J. 2008. PMID: 18498245
-
The pathogenesis of transaldolase deficiency.IUBMB Life. 2007 Jun;59(6):365-73. doi: 10.1080/15216540701387188. IUBMB Life. 2007. PMID: 17613166 Review.
Cited by
-
Time and Demand are Two Critical Dimensions of Immunometabolism: The Process of Macrophage Activation and the Pentose Phosphate Pathway.Front Immunol. 2015 Apr 8;6:164. doi: 10.3389/fimmu.2015.00164. eCollection 2015. Front Immunol. 2015. PMID: 25904920 Free PMC article. Review.
-
Centers for Mendelian Genomics: A decade of facilitating gene discovery.Genet Med. 2022 Apr;24(4):784-797. doi: 10.1016/j.gim.2021.12.005. Epub 2022 Feb 9. Genet Med. 2022. PMID: 35148959 Free PMC article. Review.
-
Apparent Acetaminophen Toxicity in a Patient with Transaldolase Deficiency.JIMD Rep. 2019;44:9-15. doi: 10.1007/8904_2018_116. Epub 2018 Jun 20. JIMD Rep. 2019. PMID: 29923087 Free PMC article.
-
mTOR-dependent loss of PON1 secretion and antiphospholipid autoantibody production underlie autoimmunity-mediated cirrhosis in transaldolase deficiency.J Autoimmun. 2023 Nov;140:103112. doi: 10.1016/j.jaut.2023.103112. Epub 2023 Sep 22. J Autoimmun. 2023. PMID: 37742509 Free PMC article.
-
Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase.Trends Mol Med. 2011 Jul;17(7):395-403. doi: 10.1016/j.molmed.2011.01.014. Epub 2011 Mar 2. Trends Mol Med. 2011. PMID: 21376665 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

