Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors
- PMID: 19436115
- PMCID: PMC2689109
- DOI: 10.1172/JCI36891
Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors
Abstract
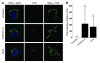
Adeno-associated virus (AAV) vectors are effective gene delivery vehicles mediating long-lasting transgene expression. Data from a clinical trial of AAV2-mediated hepatic transfer of the Factor IX gene (F9) into hemophilia B subjects suggests that CTL responses against AAV capsid can eliminate transduced hepatocytes and prevent long-term F9 expression. However, the capacity of hepatocytes to present AAV capsid-derived antigens has not been formally demonstrated, nor whether transduction by AAV sensitizes hepatocytes for CTL-mediated destruction. To investigate the fate of capsids after transduction, we engineered a soluble TCR for the detection of capsid-derived peptide:MHC I (pMHC) complexes. TCR multimers exhibited antigen and HLA specificity and possessed high binding affinity for cognate pMHC complexes. With this reagent, capsid pMHC complexes were detectable by confocal microscopy following AAV-mediated transduction of human hepatocytes. Although antigen presentation was modest, it was sufficient to flag transduced cells for CTL-mediated lysis in an in vitro killing assay. Destruction of hepatocytes was inhibited by soluble TCR, demonstrating a possible application for this reagent in blocking undesirable CTL responses. Together, these studies provide a mechanism for the loss of transgene expression and transient elevations in aminotransferases following AAV-mediated hepatic gene transfer in humans and a potential therapeutic intervention to abrogate these limitations imposed by the host T cell response.
Figures



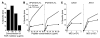
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

