Bacterial endotoxin enhances colorectal cancer cell adhesion and invasion through TLR-4 and NF-kappaB-dependent activation of the urokinase plasminogen activator system
- PMID: 19436306
- PMCID: PMC2696751
- DOI: 10.1038/sj.bjc.6604942
Bacterial endotoxin enhances colorectal cancer cell adhesion and invasion through TLR-4 and NF-kappaB-dependent activation of the urokinase plasminogen activator system
Abstract
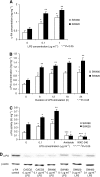
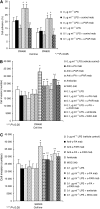
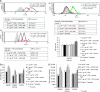
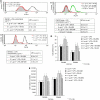
Perioperative exposure to lipopolysaccharide (LPS) is associated with accelerated metastatic colorectal tumour growth. LPS directly affects cells through Toll-like receptor 4 (TLR-4) and the transcription factor NF-kappaB. The urokinase plasminogen activator (u-PA) system is intimately implicated in tumour cell extracellular matrix (ECM) interactions fundamental to tumour progression. Thus we sought to determine if LPS directly induces accelerated tumour cell ECM adhesion and invasion through activation of the u-PA system and to elucidate the cellular pathways involved. Human colorectal tumour cell lines were stimulated with LPS. u-PA concentration, u-PA activity, active u-PA, surface urokinase plasminogen activator receptor (u-PAR) and TLR-4 expression were assessed by ELISA, colorimetric assay, western blot analysis and flow cytometry respectively. In vitro tumour cell vitronectin adhesion and ECM invasion were analysed by vitronectin adhesion assay and ECM invasion chambers. u-PA and u-PAR function was inhibited with anti u-PA antibodies or the selective u-PA inhibitors amiloride or WXC-340, TLR-4 by TLR-4-blocking antibodies and NF-kappaB by the selective NF-kappaB inhibitor SN-50. LPS upregulates u-PA and u-PAR in a dose-dependent manner, enhancing in vitro tumour cell vitronectin adhesion and ECM invasion by >40% (P<0.01). These effects were ameliorated by u-PA and u-PAR inhibition. LPS activates NF-kappaB through TLR-4. TLR-4 and NF-kappaB inhibition ameliorated LPS-enhanced u-PA and u-PAR expression, tumour cell vitronectin adhesion and ECM invasion. LPS promotes tumour cell ECM adhesion and invasion through activation of the u-PA system in a TLR-4- and NF-kappaB-dependent manner.
Figures



References
-
- Aggarwal B (2004) Nuclear factor-κB: the enemy within. Cancer Cell 6: 203–208 - PubMed
-
- Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ (2000) Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat Med 1: 100–110 - PubMed
-
- Andrews EJ, Wang JH, Winter DC, Lang WE, Redmond HP (2001) Tumor cell adhesion to endothelial cells is increased by endotoxin via an upregulation of beta-1 integrin expression. J Surg Res 97: 14–19 - PubMed
-
- Böcker U, Yezerskyy O, Feick P, Manigold T, Panja A, Kalina U, Herweck F, Rossol S, Singer MV (2003) Responsiveness of intestinal epithelial cell lines to lipopolysaccharide is correlated with Toll-like receptor 4 but not Toll-like receptor 2 or CD14 expression. Int J Colorectal Dis 18: 25–32 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

