Topical retapamulin in the management of infected traumatic skin lesions
- PMID: 19436611
- PMCID: PMC2697516
- DOI: 10.2147/tcrm.s3459
Topical retapamulin in the management of infected traumatic skin lesions
Abstract
Retapamulin is a novel semisynthetic pleuromutilin antibiotic specifically designed for use as a topical agent. The unique mode of action by which retapamulin selectively inhibits bacterial protein synthesis differentiates it from other nonpleuromutilin antibacterial agents that target the ribosome or ribosomal factors, minimizing the potential for target-specific cross-resistance with other antibacterial classes in current use. In vitro studies show that retapamulin has high potency against the Gram-positive bacteria (Staphylococcus aureus, Streptococcus pyogenes, and coagulase-negative staphylococci) commonly found in skin and skin-structure infections (SSSIs), including S. aureus strains with resistance to agents such as macrolides, fusidic acid, or mupirocin, and other less common organisms associated with SSSIs, anaerobes, and common respiratory tract pathogens. Clinical studies have shown that twice-daily topical retapamulin for 5 days is comparable to 10 days of oral cephalexin in the treatment of secondarily infected traumatic lesions. A 1% concentration of retapamulin ointment has been approved for clinical use as an easily applied treatment with a short, convenient dosing regimen for impetigo. Given the novel mode of action, low potential for cross-resistance with established antibacterial agents, and high in vitro potency against many bacterial pathogens commonly recovered from SSSIs, retapamulin is a valuable enhancement over existing therapeutic options.
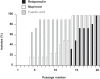
Figures



Similar articles
-
Spotlight on retapamulin in impetigo and other uncomplicated superficial skin infections.Am J Clin Dermatol. 2008;9(6):411-3. doi: 10.2165/0128071-200809060-00010. Am J Clin Dermatol. 2008. PMID: 18973410
-
Retapamulin: a review of its use in the management of impetigo and other uncomplicated superficial skin infections.Drugs. 2008;68(6):855-73. doi: 10.2165/00003495-200868060-00008. Drugs. 2008. PMID: 18416589 Review.
-
Retapamulin ointment twice daily for 5 days vs oral cephalexin twice daily for 10 days for empiric treatment of secondarily infected traumatic lesions of the skin.Skinmed. 2006 Sep-Oct;5(5):224-32. doi: 10.1111/j.1540-9740.2006.05774.x. Skinmed. 2006. PMID: 16957433 Clinical Trial.
-
Retapamulin: a new topical antibiotic for the treatment of uncomplicated skin infections.Drugs Today (Barc). 2008 Feb;44(2):91-102. doi: 10.1358/dot.2008.44.2.1153446. Drugs Today (Barc). 2008. PMID: 18389088 Review.
-
Microbiological profile of a new topical antibacterial: retapamulin ointment 1%.Expert Rev Anti Infect Ther. 2009 Apr;7(3):269-79. doi: 10.1586/eri.09.7. Expert Rev Anti Infect Ther. 2009. PMID: 19344241 Review.
Cited by
-
No decrease in susceptibility to NVC-422 in multiple-passage studies with methicillin-resistant Staphylococcus aureus, S. aureus, Pseudomonas aeruginosa, and Escherichia coli.Antimicrob Agents Chemother. 2012 May;56(5):2753-5. doi: 10.1128/AAC.05985-11. Epub 2012 Feb 21. Antimicrob Agents Chemother. 2012. PMID: 22354299 Free PMC article.
-
Cutaneous infections and infestations: new therapies.J Clin Aesthet Dermatol. 2011 Dec;4(12):18-24. J Clin Aesthet Dermatol. 2011. PMID: 22191004 Free PMC article.
-
Efficacy of Dalbavancin and Telavancin in the Treatment of Acute Bacterial Skin and Skin Structure Infections.Maedica (Bucur). 2018 Sep;13(3):208-212. doi: 10.26574/maedica.2018.13.3.208. Maedica (Bucur). 2018. PMID: 30568740 Free PMC article.
-
Review - Expert Opinion on Antibiotics and Antibiotic Resistance in Dermatology.Dermatol Pract Concept. 2024 Oct 30;14(4):e2024282. doi: 10.5826/dpc.1404a282. Dermatol Pract Concept. 2024. PMID: 39392426 Free PMC article. Review.
References
-
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–1406. - PubMed
-
- Perera G, Hay R. A guide to antibiotic resistance in bacterial skin infections. J Eur Acad Dermatol Venereol. 2005;19:531–545. - PubMed
-
- Patel M, Waites KB, Hoesley CJ, Stamm AM, Canupp KC, Moser SA. Emergence of USA300 MRSA in a tertiary medical centre: implications for epidemiological studies. J Hosp Infect. 2008;68:208–213. - PubMed
LinkOut - more resources
Full Text Sources

