Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers
- PMID: 19436712
- PMCID: PMC2674935
- DOI: 10.1371/journal.ppat.1000433
Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers
Abstract
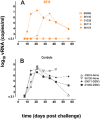
Developing an immunogen that elicits broadly neutralizing antibodies (bNAbs) is an elusive but important goal of HIV vaccine research, especially after the recent failure of the leading T cell based HIV vaccine in human efficacy trials. Even if such an immunogen can be developed, most animal model studies indicate that high serum neutralizing concentrations of bNAbs are required to provide significant benefit in typical protection experiments. One possible exception is provided by the anti-glycan bNAb 2G12, which has been reported to protect macaques against CXCR4-using SHIV challenge at relatively low serum neutralizing titers. Here, we investigated the ability of 2G12 administered intravenously (i.v.) to protect against vaginal challenge of rhesus macaques with the CCR5-using SHIV(SF162P3). The results show that, at 2G12 serum neutralizing titers of the order of 1:1 (IC(90)), 3/5 antibody-treated animals were protected with sterilizing immunity, i.e. no detectable virus replication following challenge; one animal showed a delayed and lowered primary viremia and the other animal showed a course of infection similar to 4 control animals. This result contrasts strongly with the typically high titers observed for protection by other neutralizing antibodies, including the bNAb b12. We compared b12 and 2G12 for characteristics that might explain the differences in protective ability relative to neutralizing activity. We found no evidence to suggest that 2G12 transudation to the vaginal surface was significantly superior to b12. We also observed that the ability of 2G12 to inhibit virus replication in target cells through antibody-mediated effector cell activity in vitro was equivalent or inferior to b12. The results raise the possibility that some epitopes on HIV may be better vaccine targets than others and support targeting the glycan shield of the envelope.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Johnston MI, Fauci AS. An HIV vaccine–evolving concepts. N Engl J Med. 2007;356:2073–2081. - PubMed
-
- Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320:760–764. - PubMed
-
- Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

