The F(0)F(1)-ATP synthase complex contains novel subunits and is essential for procyclic Trypanosoma brucei
- PMID: 19436713
- PMCID: PMC2674945
- DOI: 10.1371/journal.ppat.1000436
The F(0)F(1)-ATP synthase complex contains novel subunits and is essential for procyclic Trypanosoma brucei
Abstract
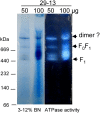
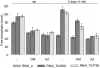
The mitochondrial F(0)F(1) ATP synthase is an essential multi-subunit protein complex in the vast majority of eukaryotes but little is known about its composition and role in Trypanosoma brucei, an early diverged eukaryotic pathogen. We purified the F(0)F(1) ATP synthase by a combination of affinity purification, immunoprecipitation and blue-native gel electrophoresis and characterized its composition and function. We identified 22 proteins of which five are related to F(1) subunits, three to F(0) subunits, and 14 which have no obvious homology to proteins outside the kinetoplastids. RNAi silencing of expression of the F(1) alpha subunit or either of the two novel proteins showed that they are each essential for the viability of procyclic (insect stage) cells and are important for the structural integrity of the F(0)F(1)-ATP synthase complex. We also observed a dramatic decrease in ATP production by oxidative phosphorylation after silencing expression of each of these proteins while substrate phosphorylation was not severely affected. Our procyclic T. brucei cells were sensitive to the ATP synthase inhibitor oligomycin even in the presence of glucose contrary to earlier reports. Hence, the two novel proteins appear essential for the structural organization of the functional complex and regulation of mitochondrial energy generation in these organisms is more complicated than previously thought.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
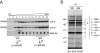




Similar articles
-
The F1 -ATPase from Trypanosoma brucei is elaborated by three copies of an additional p18-subunit.FEBS J. 2018 Feb;285(3):614-628. doi: 10.1111/febs.14364. Epub 2017 Dec 30. FEBS J. 2018. PMID: 29247468
-
The F1-ATP synthase complex in bloodstream stage trypanosomes has an unusual and essential function.EMBO J. 2005 Dec 7;24(23):4029-40. doi: 10.1038/sj.emboj.7600862. Epub 2005 Nov 17. EMBO J. 2005. PMID: 16270030 Free PMC article.
-
Disparate phenotypic effects from the knockdown of various Trypanosoma brucei cytochrome c oxidase subunits.Mol Biochem Parasitol. 2012 Aug;184(2):90-8. doi: 10.1016/j.molbiopara.2012.04.013. Epub 2012 May 5. Mol Biochem Parasitol. 2012. PMID: 22569586
-
Redesigned and reversed: architectural and functional oddities of the trypanosomal ATP synthase.Parasitology. 2021 Sep;148(10):1151-1160. doi: 10.1017/S0031182021000202. Epub 2021 Feb 8. Parasitology. 2021. PMID: 33551002 Free PMC article. Review.
-
The mitochondrial ATP synthase of Trypanosoma brucei: structure and regulation.J Bioenerg Biomembr. 1994 Apr;26(2):173-8. doi: 10.1007/BF00763066. J Bioenerg Biomembr. 1994. PMID: 8056784 Review.
Cited by
-
Deep kinetoplast genome analyses result in a novel molecular assay for detecting Trypanosoma brucei gambiense-specific minicircles.NAR Genom Bioinform. 2022 Oct 20;4(4):lqac081. doi: 10.1093/nargab/lqac081. eCollection 2022 Dec. NAR Genom Bioinform. 2022. PMID: 36285287 Free PMC article.
-
CRISPR Genome Editing and the Study of Chagas Disease.Adv Exp Med Biol. 2023;1429:111-125. doi: 10.1007/978-3-031-33325-5_7. Adv Exp Med Biol. 2023. PMID: 37486519
-
NUP-1 Is a large coiled-coil nucleoskeletal protein in trypanosomes with lamin-like functions.PLoS Biol. 2012;10(3):e1001287. doi: 10.1371/journal.pbio.1001287. Epub 2012 Mar 27. PLoS Biol. 2012. PMID: 22479148 Free PMC article.
-
Highly divergent mitochondrial ATP synthase complexes in Tetrahymena thermophila.PLoS Biol. 2010 Jul 13;8(7):e1000418. doi: 10.1371/journal.pbio.1000418. PLoS Biol. 2010. PMID: 20644710 Free PMC article.
-
Gene expression to mitochondrial metabolism: Variability among cultured Trypanosoma cruzi strains.PLoS One. 2018 May 30;13(5):e0197983. doi: 10.1371/journal.pone.0197983. eCollection 2018. PLoS One. 2018. PMID: 29847594 Free PMC article.
References
-
- Bringaud F, Riviere L, Coustou V. Energy metabolism of trypanosomatids: adaptation to available carbon sources. Mol Biochem Parasitol. 2006;149:1–9. - PubMed
-
- Besteiro S, Barrett MP, Riviere L, Bringaud F. Energy generation in insect stages of Trypanosoma brucei: metabolism in flux. Trends Parasitol. 2005;21:185–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

