De novo generation of infectious prions in vitro produces a new disease phenotype
- PMID: 19436715
- PMCID: PMC2675078
- DOI: 10.1371/journal.ppat.1000421
De novo generation of infectious prions in vitro produces a new disease phenotype
Erratum in
- PLoS Pathog. 2013 Mar;9(3). doi:10.1371/annotation/4b55946a-edb5-4feb-aeb5-2ee160394d17
Abstract
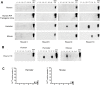
Prions are the proteinaceous infectious agents responsible for Transmissible Spongiform Encephalopathies. Compelling evidence supports the hypothesis that prions are composed exclusively of a misfolded version of the prion protein (PrP(Sc)) that replicates in the body in the absence of nucleic acids by inducing the misfolding of the cellular prion protein (PrP(C)). The most common form of human prion disease is sporadic, which appears to have its origin in a low frequency event of spontaneous misfolding to generate the first PrP(Sc) particle that then propagates as in the infectious form of the disease. The main goal of this study was to mimic an early event in the etiology of sporadic disease by attempting de novo generation of infectious PrP(Sc)in vitro. For this purpose we analyzed in detail the possibility of spontaneous generation of PrP(Sc) by the protein misfolding cyclic amplification (PMCA) procedure. Under standard PMCA conditions, and taking precautions to avoid cross-contamination, de novo generation of PrP(Sc) was never observed, supporting the use of the technology for diagnostic applications. However, we report that PMCA can be modified to generate PrP(Sc) in the absence of pre-existing PrP(Sc) in different animal species at a low and variable rate. De novo generated PrP(Sc) was infectious when inoculated into wild type hamsters, producing a new disease phenotype with unique clinical, neuropathological and biochemical features. Our results represent additional evidence in support of the prion hypothesis and provide a simple model to study the mechanism of sporadic prion disease. The findings also suggest that prion diversity is not restricted to those currently known, and that likely new forms of infectious protein foldings may be produced, resulting in novel disease phenotypes.
Conflict of interest statement
Dr. Soto is an inventor on the PMCA technology and a Founder, Vice-President and Chief Scientific Officer of Amprion Inc, a biotech company focusing on the development of early and sensitive diagnosis for prion diseases and other disorders involving protein misfolding.
Figures



References
-
- Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001;24:519–550. - PubMed
-
- Castilla J, Saá P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. - PubMed
-
- Legname G, Baskakov IV, Nguyen HO, Riesner D, Cohen FE, et al. Synthetic mammalian prions. Science. 2004;305:673–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

