Counteracting quasispecies adaptability: extinction of a ribavirin-resistant virus mutant by an alternative mutagenic treatment
- PMID: 19436746
- PMCID: PMC2677667
- DOI: 10.1371/journal.pone.0005554
Counteracting quasispecies adaptability: extinction of a ribavirin-resistant virus mutant by an alternative mutagenic treatment
Abstract
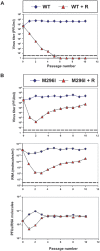
Background: Lethal mutagenesis, or virus extinction promoted by mutagen-induced elevation of mutation rates of viruses, may meet with the problem of selection of mutagen-resistant variants, as extensively documented for standard, non-mutagenic antiviral inhibitors. Previously, we characterized a mutant of foot-and-mouth disease virus that included in its RNA-dependent RNA polymerase replacement M296I that decreased the sensitivity of the virus to the mutagenic nucleoside analogue ribavirin.
Methodology and principal findings: Replacement M296I in the viral polymerase impedes the extinction of the mutant foot-and-mouth disease virus by elevated concentrations of ribavirin. In contrast, wild type virus was extinguished by the same ribavirin treatment and, interestingly, no mutants resistant to ribavirin were selected from the wild type populations. Decreases of infectivity and viral load of the ribavirin-resistant M296I mutant were attained with a combination of the mutagen 5-fluorouracil and the non-mutagenic inhibitor guanidine hydrocloride. However, extinction was achieved with a sequential treatment, first with ribavirin, and then with a minimal dose of 5-fluorouracil in combination with guanidine hydrochloride. Both, wild type and ribavirin-resistant mutant M296I exhibited equal sensitivity to this combination, indicating that replacement M296I in the polymerase did not confer a significant cross-resistance to 5-fluorouracil. We discuss these results in relation to antiviral designs based on lethal mutagenesis.
Conclusions: (i) When dominant in the population, a mutation that confers partial resistance to a mutagenic agent can jeopardize virus extinction by elevated doses of the same mutagen. (ii) A wild type virus, subjected to identical high mutagenic treatment, need not select a mutagen-resistant variant, and the population can be extinguished. (iii) Extinction of the mutagen-resistant variant can be achieved by a sequential treatment of a high dose of the same mutagen, followed by a combination of another mutagen with an antiviral inhibitor.
Conflict of interest statement
Figures


Similar articles
-
Foot-and-mouth disease virus mutant with decreased sensitivity to ribavirin: implications for error catastrophe.J Virol. 2007 Feb;81(4):2012-24. doi: 10.1128/JVI.01606-06. Epub 2006 Dec 6. J Virol. 2007. PMID: 17151116 Free PMC article.
-
Potential benefits of sequential inhibitor-mutagen treatments of RNA virus infections.PLoS Pathog. 2009 Nov;5(11):e1000658. doi: 10.1371/journal.ppat.1000658. Epub 2009 Nov 13. PLoS Pathog. 2009. PMID: 19911056 Free PMC article.
-
Ribavirin-resistant variants of foot-and-mouth disease virus: the effect of restricted quasispecies diversity on viral virulence.J Virol. 2014 Apr;88(8):4008-20. doi: 10.1128/JVI.03594-13. Epub 2014 Jan 22. J Virol. 2014. PMID: 24453363 Free PMC article.
-
Action of mutagenic agents and antiviral inhibitors on foot-and-mouth disease virus.Virus Res. 2005 Feb;107(2):183-93. doi: 10.1016/j.virusres.2004.11.008. Virus Res. 2005. PMID: 15649564 Review.
-
5-fluorouracil in lethal mutagenesis of foot-and-mouth disease virus.Future Med Chem. 2009 Jun;1(3):529-39. doi: 10.4155/fmc.09.26. Future Med Chem. 2009. PMID: 21426129 Review.
Cited by
-
Arenaviruses and lethal mutagenesis. Prospects for new ribavirin-based interventions.Viruses. 2012 Nov 6;4(11):2786-805. doi: 10.3390/v4112786. Viruses. 2012. PMID: 23202505 Free PMC article. Review.
-
A multi-step process of viral adaptation to a mutagenic nucleoside analogue by modulation of transition types leads to extinction-escape.PLoS Pathog. 2010 Aug 26;6(8):e1001072. doi: 10.1371/journal.ppat.1001072. PLoS Pathog. 2010. PMID: 20865120 Free PMC article.
-
Extinction of hepatitis C virus by ribavirin in hepatoma cells involves lethal mutagenesis.PLoS One. 2013 Aug 16;8(8):e71039. doi: 10.1371/journal.pone.0071039. eCollection 2013. PLoS One. 2013. PMID: 23976977 Free PMC article.
-
Mutagenesis-mediated virus extinction: virus-dependent effect of viral load on sensitivity to lethal defection.PLoS One. 2012;7(3):e32550. doi: 10.1371/journal.pone.0032550. Epub 2012 Mar 19. PLoS One. 2012. PMID: 22442668 Free PMC article.
-
Viral quasispecies evolution.Microbiol Mol Biol Rev. 2012 Jun;76(2):159-216. doi: 10.1128/MMBR.05023-11. Microbiol Mol Biol Rev. 2012. PMID: 22688811 Free PMC article. Review.
References
-
- Domingo E, editor. Quasispecies: Concepts and Implications for Virology. Current Topics in Microbiology and Immunology 2006
-
- Domingo E, Escarmís C, Menéndez-Arias L, Perales C, Herrera M, et al. Viral quasispecies: dynamics, interactions and pathogenesis. In: Domingo E, Parrish C, Holland JJ, editors. Origin and Evolution of Viruses. Oxford: Elsevier; 2008. pp. 87–118.
-
- Holland JJ, Spindler K, Horodyski F, Grabau E, Nichol S, et al. Rapid evolution of RNA genomes. Science. 1982;215:1577–1585. - PubMed

