Signal transduction pathways in liver and the influence of hepatitis C virus infection on their activities
- PMID: 19437557
- PMCID: PMC2682232
- DOI: 10.3748/wjg.15.2184
Signal transduction pathways in liver and the influence of hepatitis C virus infection on their activities
Abstract
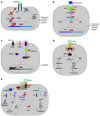
In liver, the most intensively studied transmembrane and intracellular signal transduction pathways are the Janus kinase signal transduction pathway, the mitogen-activated protein kinases signal transduction pathway, the transforming growth factor beta signal transduction pathway, the tumor necrosis factor alpha signal transduction pathway and the recently discovered sphingolipid signal transduction pathway. All of them are activated by many different cytokines and growth factors. They regulate specific cell mechanisms such as hepatocytes proliferation, growth, differentiation, adhesion, apoptosis, and synthesis and degradation of the extracellular matrix. The replication cycle of hepatitis C virus (HCV) is intracellular and requires signal transduction to the nucleus to regulate transcription of its genes. Moreover, HCV itself, by its structural and non-structural proteins, could influence the activity of the second signal messengers. Thus, the inhibition of the transmembrane and intracellular signal transduction pathways could be a new therapeutic target in chronic hepatitis C treatment.
Figures
References
-
- Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. - PubMed
-
- Lindenbach BD, Rice CM. Flaviviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 4th edition, volume 1. Philadelphia: Lippincott-Raven Publishers; 2001. pp. 991–1041.
-
- Czepiel J, Biesiada G, Mach T. Viral hepatitis C. Pol Arch Med Wewn. 2008;118:734–740. - PubMed
-
- Shuai K. Regulation of cytokine signaling pathways by PIAS proteins. Cell Res. 2006;16:196–202. - PubMed
-
- Krebs DL, Hilton DJ. SOCS: physiological suppressors of cytokine signaling. J Cell Sci. 2000;113:2813–2819. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

