Rhodium-catalyzed C-C bond formation via heteroatom-directed C-H bond activation
- PMID: 19438203
- PMCID: PMC2820156
- DOI: 10.1021/cr900005n
Rhodium-catalyzed C-C bond formation via heteroatom-directed C-H bond activation
Figures


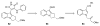






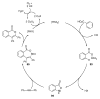
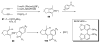
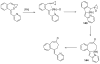
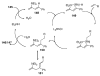






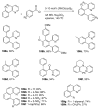

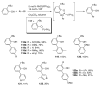
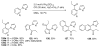
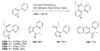



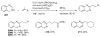


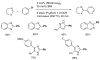
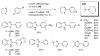




References
-
-
For reviews on C-H bond functionalization, see the following and leading references therein: Dyker G. Angew Chem Int Ed. 1999;38:1698.Kakiuchi F, Murai S. Top Organomet Chem. 1999;3:47.Ritleng V, Sirlin C, Pfeffer M. Chem Rev. 2002;102:1731.Jun CH, Moon CW, Lee H, Lee DY. J Mol Cat A. 2002;189:145.Kakiuchi F, Murai S. Acc Chem Res. 2002;35:826.Miura M, Nomura M. Top Curr Chem. 2002;219:212.Kakiuchi F, Chatani N. Adv Synth Catal. 2003;345:1077.Park YJ, Jun CH. Bull Korean Chem Soc. 2005;26:871.Kakiuchi F. Top Organomet Chem. 2007;24:1.Seregin IV, Gevorgyan V. Chem Soc Rev. 2007;36:1173.Lewis JC, Bergman RG, Ellman JA. Acc Chem Res. 2008;41:1013.Kakiuchi F, Kochi T. Synthesis. 2008:3013.
-
-
- de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. Wiley-VCH; Weinheim: 2004.
-
-
For examples of sterically-driven selectivity, see: Cho J-Y, Iverson CN, Smith MR. J Am Chem Soc. 2000;122:12868.Ishiyama T, Takagi J, Kousaku I, Miyaura N, Anastasi NR, Hartwig JF. J Am Chem Soc. 2002;124:390.For examples of selectivity based on C-H acidity, see: Lafrance M, Rowley CN, Woo TK, Fagnou K. J Am Chem Soc. 2006;128:8754.Garcia-Cuadrado D, Braga AAC, Maseras F, Echavarren AM. J Am Chem Soc. 2006;128:1066.For examples of selectivity based on electron-donating substituents, see: Jia CG, Piao DG, Oyamada JZ, Lu WJ, Kitamura T, Fujiwara Y. Science. 2000;287:1992.Tunge JA, Foresee LN. Organometallics. 2005;24:6440.Stuart DR, Villemure E, Fagnou K. J Am Chem Soc. 2007;129:12072.Stuart DR, Fagnou K. Science. 2007;316:1172.
-
-
-
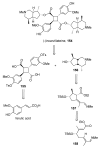
For examples of the application of C-H bond activation in target-oriented synthesis, see: Harris PWR, Woodgate PD. J Organomet Chem. 1997;530:211.Johnson JA, Sames D. J Am Chem Soc. 2000;122:6321.When PM, DuBois J. J Am Chem Soc. 2002;124:12950.Hinman A, DuBois JH. J Am Chem Soc. 2003;125:11510.Leblanc M, Fagnou K. Org Lett. 2005;7:2849.Davies HML, Dai X, Long MS. J Am Chem Soc. 2006;128:2485.Liu Y, Xiao W, Wong MK, Che CM. Org Lett. 2007;9:4107.For reviews see ref 26a and: Godula K, Sames D. Nature. 2006;312:67.Lafrance M, Blaquiere N, Fagnou K. Eur J Org Chem. 2007:811.
-
-
- Murai S, Kakiuchi F, Sekine S, Tanaka Y, Kamatani A, Sonoda M, Chatani N. Nature. 1993;366:529.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

