Co-culture of intestinal epithelial and stromal cells in 3D collagen-based environments
- PMID: 19438315
- PMCID: PMC2869023
- DOI: 10.2217/rme.09.4
Co-culture of intestinal epithelial and stromal cells in 3D collagen-based environments
Abstract
Aim: To investigate the co-culture of established intestinal epithelial cell lines and stromal cells in a series of collagen-based environments for production of tissue-engineered intestinal epithelium for in vitro investigations.
Materials & methods: Intestinal epithelial cells were co-cultured with fibroblasts on a range of supporting collagen matrices including commercially available Promogran and on collagen-based gels.
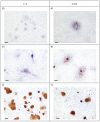
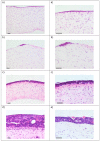
Results: Epithelial growth was achieved with one combination of vimentin-expressing stromal and cytokeratin-expressing intestinal epithelial cells grown on collagen gels supplemented with Matrigel, and held at an air-liquid interface.
Conclusions: Collagen-based gels can support the co-culture of intestinal epithelial and stromal cells resulting in the growth of an epithelium that has some morphological similarity to normal intestinal tissue.
Figures



Similar articles
-
Type I collagen as an extracellular matrix for the in vitro growth of human small intestinal epithelium.PLoS One. 2014 Sep 15;9(9):e107814. doi: 10.1371/journal.pone.0107814. eCollection 2014. PLoS One. 2014. PMID: 25222024 Free PMC article.
-
Two- and Three-Dimensional Bioengineered Human Intestinal Tissue Models for Cryptosporidium.Methods Mol Biol. 2020;2052:373-402. doi: 10.1007/978-1-4939-9748-0_21. Methods Mol Biol. 2020. PMID: 31452173 Free PMC article.
-
Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model.Hum Reprod. 2001 May;16(5):836-45. doi: 10.1093/humrep/16.5.836. Hum Reprod. 2001. PMID: 11331626
-
Materials and Microenvironments for Engineering the Intestinal Epithelium.Ann Biomed Eng. 2020 Jul;48(7):1916-1940. doi: 10.1007/s10439-020-02470-8. Epub 2020 Feb 4. Ann Biomed Eng. 2020. PMID: 32020347 Review.
-
Intestinal epithelial cells in vitro.Stem Cells Dev. 2010 Jan;19(1):131-42. doi: 10.1089/scd.2009.0109. Stem Cells Dev. 2010. PMID: 19580443 Free PMC article. Review.
Cited by
-
Comparison of conventional and advanced in vitro models in the toxicity testing of nanoparticles.Artif Cells Nanomed Biotechnol. 2018;46(sup2):1091-1107. doi: 10.1080/21691401.2018.1479709. Epub 2018 Jun 29. Artif Cells Nanomed Biotechnol. 2018. PMID: 29956556 Free PMC article. Review.
-
Buckling of a growing tissue and the emergence of two-dimensional patterns.Math Biosci. 2013 Dec;246(2):229-41. doi: 10.1016/j.mbs.2013.09.008. Epub 2013 Oct 12. Math Biosci. 2013. PMID: 24128749 Free PMC article.
-
Comparison of polyglycolic acid, polycaprolactone, and collagen as scaffolds for the production of tissue engineered intestine.J Biomed Mater Res B Appl Biomater. 2019 Apr;107(3):750-760. doi: 10.1002/jbm.b.34169. Epub 2018 Sep 30. J Biomed Mater Res B Appl Biomater. 2019. PMID: 30270503 Free PMC article.
-
Pituitary Remodeling Throughout Life: Are Resident Stem Cells Involved?Front Endocrinol (Lausanne). 2021 Jan 29;11:604519. doi: 10.3389/fendo.2020.604519. eCollection 2020. Front Endocrinol (Lausanne). 2021. PMID: 33584539 Free PMC article. Review.
-
Fibroblasts modulate epithelial cell behavior within the proliferative niche and differentiated cell zone within a human colonic crypt model.Front Bioeng Biotechnol. 2024 Dec 16;12:1506976. doi: 10.3389/fbioe.2024.1506976. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39737053 Free PMC article.
References
-
- Gabe SM, Day RM, Boccaccini A. Tissue engineering of the small intestine. In: Bowlin GL, Wnek G, editors. Encyclopaedia of Biomaterials and Biomedical engineering. Marcel Dekker Inc.; New York, USA: 2004. pp. 1661–1671.
-
- Gardner-Thorpe J, Grikscheit TC, Ito H, et al. Angiogenesis in tissue-engineered small intestine. Tissue Eng. 2003;9:1255–1261. - PubMed
-
- Gupta A, Dixit A, Sales KM, Winslet MC, Seifalian AM. Tissue engineering of small intestine – current status. Biomacromolecules. 2006;7:2701–2709. - PubMed
-
- Evans GS, Flint N, Somers AS, Eyden B, Potten CS. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J. Cell. Sci. 1992;101:219–231. - PubMed
-
- Fukamachi H. Proliferation and differentiation of fetal rat intestinal epithelial cells in primary serum-free culture. J. Cell. Sci. 1992;103:511–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
