A white matter stroke model in the mouse: axonal damage, progenitor responses and MRI correlates
- PMID: 19439360
- PMCID: PMC4697458
- DOI: 10.1016/j.jneumeth.2009.03.017
A white matter stroke model in the mouse: axonal damage, progenitor responses and MRI correlates
Abstract
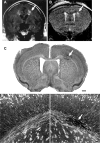
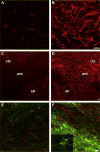
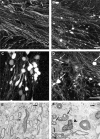
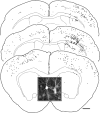
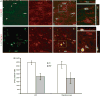
Subcortical white matter stroke is a common stroke subtype but has had limited pre-clinical modeling. Recapitulating this disease process in mice has been impeded by the relative inaccessibility of the subcortical white matter arterial supply to induce white matter ischemia in isolation. In this report, we detail a subcortical white matter stroke model developed in the mouse and its characterization with a comprehensive set of MRI, immunohistochemical, neuronal tract tracing and electron microscopic studies. Focal injection of the vasoconstrictor endothelin-1 into the subcortical white matter produces an infarct core that develops a maximal MRI signal by day 2, which is comparable in relative size and location to human subcortical stroke. Immunohistochemical studies indicate that oligodendrocyte apoptosis is maximal at day 1 and apoptotic cells extend away from the stroke core into the peri-infarct white matter. The amount of myelin loss exceeds axonal fiber loss in this peri-infarct region. Activation of microglia/macrophages takes place at 1 day after injection near injured axons. Neuronal tract tracing demonstrates that subcortical white matter stroke disconnects a large region of bilateral sensorimotor cortex. There is a robust glial response after stroke by BrdU pulse-labeling, and oligodendrocyte precursor cells are initiated to proliferate and differentiate within the first week of injury. These results demonstrate the utility of the endothelin-1 mediated subcortical stroke in the mouse to study post-stroke repair mechanisms, as the infarct core extends through the partially damaged peri-infarct white matter and induces an early glial progenitor response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Back SA, Tuohy TM, Chen H, Wallingford N, Craig A, Struve J, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–72. - PubMed
-
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

