Myosin II regulates extension, growth and patterning in the mammalian cochlear duct
- PMID: 19439495
- PMCID: PMC2685721
- DOI: 10.1242/dev.030718
Myosin II regulates extension, growth and patterning in the mammalian cochlear duct
Abstract
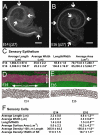
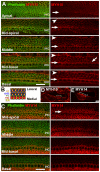
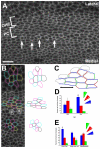
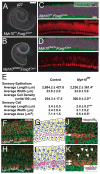
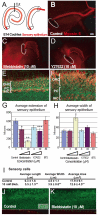
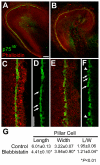
The sensory epithelium of the mammalian cochlea comprises mechanosensory hair cells that are arranged into four ordered rows extending along the length of the cochlear spiral. The factors that regulate the alignment of these rows are unknown. Results presented here demonstrate that cellular patterning within the cochlea, including the formation of ordered rows of hair cells, arises through morphological remodeling that is consistent with the mediolateral component of convergent extension. Non-muscle myosin II is shown to be expressed in a pattern that is consistent with an active role in cellular remodeling within the cochlea, and genetic or pharmacological inhibition of myosin II results in defects in cellular patterning that are consistent with a disruption in convergence and extension. These results identify the first molecule, myosin II, which directly regulates cellular patterning and alignment within the cochlear sensory epithelium. Our results also provide insights into the cellular mechanisms that are required for the formation of highly ordered cellular patterns.
Figures

References
-
- Amano, M., Ito, M., Kimura, K., Fukata, Y., Chihara, K., Nakano, T., Matsuura, Y. and Kaibuchi, K. (1996). Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, 20246-20249. - PubMed
-
- Bertet, C., Sulak, L. and Lecuit, T. (2004). Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667-671. - PubMed
-
- Bessard, A., Coutant, A., Rescan, C., Ezan, F., Fremin, C., Courselaud, B., Ilyin, G. and Baffet, G. (2006). An MLCK-dependent window in late G1 controls S phase entry of proliferating rodent hepatocytes via ERK-p70S6K pathway. Hepatology 44, 152-163. - PubMed
-
- Bok, J., Dolson, D. K., Hill, P., Ruther, U., Epstein, D. J. and Wu, D. K. (2007). Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear. Development 134, 1713-1722. - PubMed
-
- Campbell, C., Cucci, R. A., Prasad, S., Green, G. E., Edeal, J. B., Galer, C. E., Karniski, L. P., Sheffield, V. C. and Smith, R. J. (2001). Pendred syndrome, DFNB4, and PDS/SLC26A4 identification of eight novel mutations and possible genotype-phenotype correlations. Hum. Mutat. 17, 403-411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

