Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t
- PMID: 19439651
- PMCID: PMC2688993
- DOI: 10.1073/pnas.0900621106
Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t
Abstract
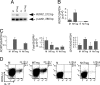
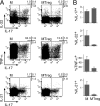
Recent studies have suggested a close relationship between CD4(+)FOXP3(+) regulatory T cells (Tregs) and proinflammatory IL-17-producing T helper cells (T(H)17) expressing the lineage-specific transcription factor RORgamma t. We report here the unexpected finding that human memory Tregs secrete IL-17 ex vivo and constitutively express RORgamma t. IL-17-secreting Tregs share some phenotypic and functional features with conventional T(H)17 cells, expressing high levels of CCR4 and CCR6 and low levels of CXCR3. However, unlike conventional T(H)17 cells, they express low levels of CD161 and mostly fail to cosecrete IL-22 and TNF-alpha ex vivo. Ex vivo secretion of IL-17 and constitutive expression of RORgamma t by human memory Tregs suggest that, in addition to their well-known suppressive functions, these cells likely play additional, as yet undescribed, proinflammatory functions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


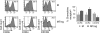
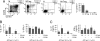
References
-
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. - PubMed
-
- Mosmann TR, Coffman RL. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. - PubMed
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. - PubMed
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

