Fatal case of deer tick virus encephalitis
- PMID: 19439744
- PMCID: PMC2847876
- DOI: 10.1056/NEJMoa0806326
Fatal case of deer tick virus encephalitis
Abstract
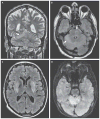
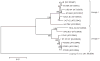
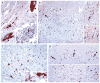
Deer tick virus is related to Powassan virus, a tickborne encephalitis virus. A 62-year-old man presented with a meningoencephalitis syndrome and eventually died. Analyses of tissue samples obtained during surgery and at autopsy revealed a widespread necrotizing meningoencephalitis. Nucleic acid was extracted from formalin-fixed tissue, and the presence of deer tick virus was verified on a flavivirus-specific polymerase-chain-reaction (PCR) assay, followed by sequence confirmation. Immunohistochemical analysis with antisera specific for deer tick virus identified numerous immunoreactive neurons, with prominent involvement of large neurons in the brain stem, cerebellum, basal ganglia, thalamus, and spinal cord. This case demonstrates that deer tick virus can be a cause of fatal encephalitis.
2009 Massachusetts Medical Society
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

References
-
- Kuno G, Artsob H, Karabatos N, Tsuchiya KR, Chang GJJ. Genomic sequencing of deer tick virus and phylogeny of Powassan-related viruses of North America. Am J Trop Med Hyg. 2001;65:671–6. - PubMed
-
- Beasley DWC, Suderman MT, Holbrook MR, Barrett ADT. Nucleotide sequencing and serological evidence that the recently recognized deer tick virus is a genotype of Powassan virus. Virus Res. 2001;79:81–9. - PubMed
-
- Ebel GD, Spielman A, Telford SR., III Phylogeny of North American Powassan virus. J Gen Virol. 2001;82:1657–65. - PubMed
-
- Charrel RN, Attoui H, Butenko AM, et al. Tick-borne virus diseases of human interest in Europe. Clin Microbiol Infect. 2004;10:1040–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
