p21 in cancer: intricate networks and multiple activities
- PMID: 19440234
- PMCID: PMC2722839
- DOI: 10.1038/nrc2657
p21 in cancer: intricate networks and multiple activities
Abstract
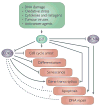
One of the main engines that drives cellular transformation is the loss of proper control of the mammalian cell cycle. The cyclin-dependent kinase inhibitor p21 (also known as p21WAF1/Cip1) promotes cell cycle arrest in response to many stimuli. It is well positioned to function as both a sensor and an effector of multiple anti-proliferative signals. This Review focuses on recent advances in our understanding of the regulation of p21 and its biological functions with emphasis on its p53-independent tumour suppressor activities and paradoxical tumour-promoting activities, and their implications in cancer.
Figures



References
-
- Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol. 2007;19:238–245. - PubMed
-
- Eastman A. Cell cycle checkpoints and their impact on anticancer therapeutic strategies. J Cell Biochem. 2004;91:223–231. - PubMed
-
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. - PubMed
-
- Brugarolas J, et al. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

