Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses
- PMID: 19440245
- PMCID: PMC2678264
- DOI: 10.1371/journal.pone.0005547
Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses
Abstract
Background: Zaire ebolavirus (ZEBOV) produces a lethal viral hemorrhagic fever in humans and non-human primates.
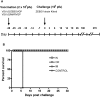
Methodology/principal findings: We demonstrate that the VSVDeltaG/ZEBOVGP vaccine given 28 days pre-challenge either intranasally (IN), orally (OR), or intramuscularly (IM) protects non-human primates against a lethal systemic challenge of ZEBOV, and induces cellular and humoral immune responses. We demonstrated that ZEBOVGP-specific T-cell and humoral responses induced in the IN and OR groups, following an immunization and challenge, produced the most IFN-gamma and IL-2 secreting cells, and long term memory responses.
Conclusions/significance: We have shown conclusively that mucosal immunization can protect from systemic ZEBOV challenge and that mucosal delivery, particularly IN immunization, seems to be more potent than IM injection in the immune parameters we have tested. Mucosal immunization would be a huge benefit in any emergency mass vaccination campaign during a natural outbreak, or following intentional release, or for mucosal immunization of great apes in the wild.
Conflict of interest statement
Figures

References
-
- Feldmann H, Jones S, Klenk HD, Schnittler HJ. Ebola virus: From discovery to vaccine. Nat Rev Immunol. 2003;3(8):677–685. - PubMed
-
- Geisbert TW, Jahrling PB. Exotic emerging viral diseases: Progress and challenges. Nat Med. 2004;10(12 Suppl):S110–21. - PubMed
-
- [Anonymous] Outbreak news. ebola haemorrhagic fever, uganda–end of the outbreak. Wkly Epidemiol Rec. 2008;83(10):89–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

