Bacterial degradation of aromatic compounds
- PMID: 19440284
- PMCID: PMC2672333
- DOI: 10.3390/ijerph6010278
Bacterial degradation of aromatic compounds
Abstract
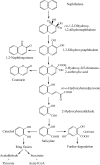
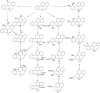
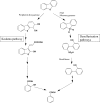
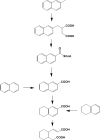
Aromatic compounds are among the most prevalent and persistent pollutants in the environment. Petroleum-contaminated soil and sediment commonly contain a mixture of polycyclic aromatic hydrocarbons (PAHs) and heterocyclic aromatics. Aromatics derived from industrial activities often have functional groups such as alkyls, halogens and nitro groups. Biodegradation is a major mechanism of removal of organic pollutants from a contaminated site. This review focuses on bacterial degradation pathways of selected aromatic compounds. Catabolic pathways of naphthalene, fluorene, phenanthrene, fluoranthene, pyrene, and benzo[a]pyrene are described in detail. Bacterial catabolism of the heterocycles dibenzofuran, carbazole, dibenzothiophene, and dibenzodioxin is discussed. Bacterial catabolism of alkylated PAHs is summarized, followed by a brief discussion of proteomics and metabolomics as powerful tools for elucidation of biodegradation mechanisms.
Keywords: Bioremediation; PAHs; biodegradation; polycyclic aromatic hydrocarbons.
Figures




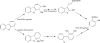

References
-
- Alexander M. Biodegradation and Bioremediation. 2nd Ed. Academic Press: San Diego, USA; 1999. pp. 325–327.
-
- Klein J. Biotechnology. Environmental Processes II – Soil Decontamination. 2nd Ed. volume 11b. Wiley-VCH: Weinhein; 2000.
-
- Cerniglia CE. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation. 1992;3:351–368.
-
- Menzie CA, Potochi BB, Santodonato J. Exposure to carcinogenic PAHs in the environment. Environ. Sci. Technol. 1992;26:1278–1284.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

