Identification of 2-aminothiazole-4-carboxylate derivatives active against Mycobacterium tuberculosis H37Rv and the beta-ketoacyl-ACP synthase mtFabH
- PMID: 19440303
- PMCID: PMC2680598
- DOI: 10.1371/journal.pone.0005617
Identification of 2-aminothiazole-4-carboxylate derivatives active against Mycobacterium tuberculosis H37Rv and the beta-ketoacyl-ACP synthase mtFabH
Abstract
Background: Tuberculosis (TB) is a disease which kills two million people every year and infects approximately over one-third of the world's population. The difficulty in managing tuberculosis is the prolonged treatment duration, the emergence of drug resistance and co-infection with HIV/AIDS. Tuberculosis control requires new drugs that act at novel drug targets to help combat resistant forms of Mycobacterium tuberculosis and reduce treatment duration.
Methodology/principal findings: Our approach was to modify the naturally occurring and synthetically challenging antibiotic thiolactomycin (TLM) to the more tractable 2-aminothiazole-4-carboxylate scaffold to generate compounds that mimic TLM's novel mode of action. We report here the identification of a series of compounds possessing excellent activity against M. tuberculosis H(37)R(v) and, dissociatively, against the beta-ketoacyl synthase enzyme mtFabH which is targeted by TLM. Specifically, methyl 2-amino-5-benzylthiazole-4-carboxylate was found to inhibit M. tuberculosis H(37)R(v) with an MIC of 0.06 microg/ml (240 nM), but showed no activity against mtFabH, whereas methyl 2-(2-bromoacetamido)-5-(3-chlorophenyl)thiazole-4-carboxylate inhibited mtFabH with an IC(50) of 0.95+/-0.05 microg/ml (2.43+/-0.13 microM) but was not active against the whole cell organism.
Conclusions/significance: These findings clearly identify the 2-aminothiazole-4-carboxylate scaffold as a promising new template towards the discovery of a new class of anti-tubercular agents.
Conflict of interest statement
Figures
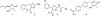

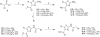


References
-
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis estimated incidence, prevalence, and mortality by country. JAMA. 1999;282:677–686. - PubMed
-
- Bloom BR, Small PM. The evolving relation between humans and mycobacterium tuberculosis. N Engl J Med. 1998;338:677–678. - PubMed
-
- Mitchison DA. The search for new sterilizing anti-tuberculosis drugs. Front Biosci. 2004;9:1059–1072. - PubMed
-
- Selwyn PA, Sckell BM, Alcabes P, Friedland GH, Klein RS, et al. High risk of active tuberculosis in HIV-infected drug users with cutaneous anergy. JAMA. 1992;268:504–509. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

