Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial
- PMID: 19440326
- PMCID: PMC2680972
- DOI: 10.1371/journal.pone.0005575
Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial
Abstract
Background: Optimal timing of ART initiation for individuals presenting with AIDS-related OIs has not been defined.
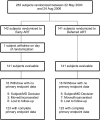
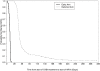
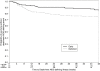
Methods and findings: A5164 was a randomized strategy trial of "early ART"--given within 14 days of starting acute OI treatment versus "deferred ART"--given after acute OI treatment is completed. Randomization was stratified by presenting OI and entry CD4 count. The primary week 48 endpoint was 3-level ordered categorical variable: 1. Death/AIDS progression; 2. No progression with incomplete viral suppression (ie HIV viral load (VL) >or=50 copies/ml); 3. No progression with optimal viral suppression (ie HIV VL <50 copies/ml). Secondary endpoints included: AIDS progression/death; plasma HIV RNA and CD4 responses and safety parameters including IRIS. 282 subjects were evaluable; 141 per arm. Entry OIs included Pneumocytis jirovecii pneumonia 63%, cryptococcal meningitis 12%, and bacterial infections 12%. The early and deferred arms started ART a median of 12 and 45 days after start of OI treatment, respectively. THE DIFFERENCE IN THE PRIMARY ENDPOINT DID NOT REACH STATISTICAL SIGNIFICANCE: AIDS progression/death was seen in 20 (14%) vs. 34 (24%); whereas no progression but with incomplete viral suppression was seen in 54 (38%) vs. 44 (31%); and no progression with optimal viral suppression in 67 (48%) vs 63 (45%) in the early vs. deferred arm, respectively (p = 0.22). However, the early ART arm had fewer AIDS progression/deaths (OR = 0.51; 95% CI = 0.27-0.94) and a longer time to AIDS progression/death (stratified HR = 0.53; 95% CI = 0.30-0.92). The early ART had shorter time to achieving a CD4 count above 50 cells/mL (p<0.001) and no increase in adverse events.
Conclusions: Early ART resulted in less AIDS progression/death with no increase in adverse events or loss of virologic response compared to deferred ART. These results support the early initiation of ART in patients presenting with acute AIDS-related OIs, absent major contraindications.
Trial registration: ClinicalTrials.gov NCT00055120.
Conflict of interest statement
Figures


References
-
- Palella FJ, Baker RK, Moorman AC, Chmiel JS, Wood KC, et al. Mortality in the highly active antiretroviral therapy era. J Acquir Immune Defic Syndr. 2006;43(1):27–34. - PubMed
-
- Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. The Lancet. 2003;362 (9377):2–29. - PubMed
-
- Lewden C, Chene G, Morlat P, Raffi F, Dupon M, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach mortality rates as the general population. J Acquir Immune Defic Syndr. 2007;46(1):72–77. - PubMed
-
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337(11):725–33. - PubMed
-
- Hirsch M, Steigbigel R, Staszewski S, Scerpella E, Hirschel B, et al. A randomized, controlled trial of indinavir, zidovudine, and lamivudine in adults with advanced immunodeficiency virus type 1 infection and prior antiretroviral therapy. JID. 1999;180(3):659–665. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

