Functional profiling reveals critical role for miRNA in differentiation of human mesenchymal stem cells
- PMID: 19440384
- PMCID: PMC2680014
- DOI: 10.1371/journal.pone.0005605
Functional profiling reveals critical role for miRNA in differentiation of human mesenchymal stem cells
Abstract
Background: Mesenchymal stem (MS) cells are excellent candidates for cell-based therapeutic strategies to regenerate injured tissue. Although human MS cells can be isolated from bone marrow and directed to differentiate by means of an osteogenic pathway, the regulation of cell-fate determination is not well understood. Recent reports identify critical roles for microRNAs (miRNAs), regulators of gene expression either by inhibiting the translation or by stimulating the degradation of target mRNAs.
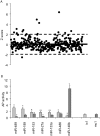
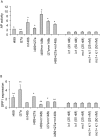
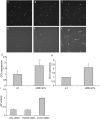
Methodology/principal findings: In this study, we employed a library of miRNA inhibitors to evaluate the role of miRNAs in early osteogenic differentiation of human MS cells. We discovered that miR-148b, -27a and -489 are essential for the regulation of osteogenesis: miR-27a and miR-489 down-regulate while miR-148b up-regulates differentiation. Modulation of these miRNAs induced osteogenesis in the absence of other external differentiation cues and restored osteogenic potential in high passage number human MS cells.
Conclusions/significance: Overall, we have demonstrated the utility of the functional profiling strategy for unraveling complex miRNA pathways. Our findings indicate that miRNAs regulate early osteogenic differentiation in human MS cells: miR-148b, -27a, and -489 were found to play a critical role in osteogenesis.
Conflict of interest statement
Figures

Similar articles
-
MiR-27a is Essential for the Shift from Osteogenic Differentiation to Adipogenic Differentiation of Mesenchymal Stem Cells in Postmenopausal Osteoporosis.Cell Physiol Biochem. 2016;39(1):253-65. doi: 10.1159/000445621. Epub 2016 Jun 24. Cell Physiol Biochem. 2016. PMID: 27337099
-
miR-27a attenuates adipogenesis and promotes osteogenesis in steroid-induced rat BMSCs by targeting PPARγ and GREM1.Sci Rep. 2016 Dec 2;6:38491. doi: 10.1038/srep38491. Sci Rep. 2016. PMID: 27910957 Free PMC article.
-
MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31.Gene. 2013 Sep 15;527(1):321-31. doi: 10.1016/j.gene.2013.06.021. Epub 2013 Jul 1. Gene. 2013. PMID: 23827457
-
Roles for miRNAs in osteogenic differentiation of bone marrow mesenchymal stem cells.Stem Cell Res Ther. 2019 Jun 28;10(1):197. doi: 10.1186/s13287-019-1309-7. Stem Cell Res Ther. 2019. PMID: 31253175 Free PMC article. Review.
-
Concise review: MicroRNA function in multipotent mesenchymal stromal cells.Stem Cells. 2014 May;32(5):1074-82. doi: 10.1002/stem.1623. Stem Cells. 2014. PMID: 24860868 Free PMC article. Review.
Cited by
-
Next generation bone tissue engineering: non-viral miR-133a inhibition using collagen-nanohydroxyapatite scaffolds rapidly enhances osteogenesis.Sci Rep. 2016 Jun 14;6:27941. doi: 10.1038/srep27941. Sci Rep. 2016. PMID: 27297802 Free PMC article.
-
A microRNA signature associated with chondrogenic lineage commitment.J Genet. 2012 Aug;91(2):171-82. doi: 10.1007/s12041-012-0168-0. J Genet. 2012. PMID: 22942087
-
Mechanosensitive miR-99b mediates the regulatory effect of matrix stiffness on bone marrow mesenchymal stem cell fate both in vitro and in vivo.APL Bioeng. 2023 Jan 18;7(1):016106. doi: 10.1063/5.0131125. eCollection 2023 Mar. APL Bioeng. 2023. PMID: 36685676 Free PMC article.
-
miR-489 is a tumour-suppressive miRNA target PTPN11 in hypopharyngeal squamous cell carcinoma (HSCC).Br J Cancer. 2010 Sep 7;103(6):877-84. doi: 10.1038/sj.bjc.6605811. Epub 2010 Aug 10. Br J Cancer. 2010. PMID: 20700123 Free PMC article.
-
Collagen-infilled 3D printed scaffolds loaded with miR-148b-transfected bone marrow stem cells improve calvarial bone regeneration in rats.Mater Sci Eng C Mater Biol Appl. 2019 Dec;105:110128. doi: 10.1016/j.msec.2019.110128. Epub 2019 Aug 24. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31546389 Free PMC article.
References
-
- Friedenstein A, Kuralesova AI. Osteogenic precursor cells of bone marrow in radiation chimeras. Transplantation. 1971;12:99–108. - PubMed
-
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. - PubMed
-
- Butler WT. The nature and significance of osteopontin. Connect Tissue Res. 1989;23:123–136. - PubMed
-
- Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. - PubMed
-
- Bentwich I. Identifying human microRNAs. Curr Top Microbiol Immunol. 2008;320:257–269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

