Time- and dose-related effects of di-(2-ethylhexyl) phthalate and its main metabolites on the function of the rat fetal testis in vitro
- PMID: 19440488
- PMCID: PMC2679593
- DOI: 10.1289/ehp.11870
Time- and dose-related effects of di-(2-ethylhexyl) phthalate and its main metabolites on the function of the rat fetal testis in vitro
Abstract
Background: Endocrine-disrupting effects of phthalates are understood primarily from in utero exposures within the fetal rat testis. Nevertheless, their path of action, dose-response character, and cellular target(s) within the fetal testis are not known.
Objectives: In this study we investigated the effects of di-(2-ethylhexyl) phthalate (DEHP), mono-(2-ethylhexyl) phthalate (MEHP), and several of their metabolites on the development of organo-cultured testes from rat fetus.
Methods: We removed testes from 14.5-day-old rat fetuses and cultured them for 1-3 days with or without DEHP, MEHP, and the metabolites.
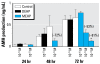
Results: DEHP (10(-5) M) produced a proandrogenic effect after 3 days of culture, whereas MEHP disrupted testis morphology and function. Leydig cells were the first affected by MEHP, with a number of them being inappropriately located within some seminiferous tubules. Additionally, we found a time- and dose-dependent reduction of testosterone. By 48 hr, gonocyte proliferation had decreased, whereas apoptosis increased. Sertoli cell number was unaffected, although some cells appeared vacuolated, and production of anti-Müllerian hormone decreased in a time- and dose-dependent manner. The derived metabolite mono-(2-ethyl-5-hydroxyhexyl) phthalate was the only one to cause deleterious effects to the rat fetal testis in vitro.
Conclusion: We hope that this in vitro method will facilitate the study of different phthalate esters and other endocrine disruptors for direct testicular effects.
Keywords: androgens; anti-Müllerian hormone; endocrine disruption; explant culture; fetal testis; gonocytes; phthalates.
Figures







References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:238–248. - PubMed
-
- ATSDR. Toxicological Profile for Diethyl Phthalate. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2002. [[accessed 18 February 2009]]. Available: http://www.atsdr.cdc.gov/toxprofiles/tp73.html. - PubMed
-
- Barlow NJ, Phillips SL, Wallace DG, Sar M, Gaido KW, Foster PM. Quantitative changes in gene expression in fetal rat testes following exposure to di(n-butyl) phthalate. Toxicol Sci. 2003;73(2):431–441. - PubMed
-
- Becker K, Seiwert M, Angerer J, Heger W, Koch HM, Nagorka R, et al. DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health. 2004;207(5):409–417. - PubMed
-
- Boekelheide K, Lee J, Shipp EB, Richburg JH, Li G. Expression of Fas system-related genes in the testis during development and after toxicant exposure. Toxicol Lett. 1998:102–103. 503–508. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

