Human neural stem cells genetically modified to overexpress Akt1 provide neuroprotection and functional improvement in mouse stroke model
- PMID: 19440551
- PMCID: PMC2679145
- DOI: 10.1371/journal.pone.0005586
Human neural stem cells genetically modified to overexpress Akt1 provide neuroprotection and functional improvement in mouse stroke model
Abstract
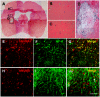
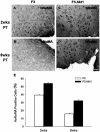
In a previous study, we have shown that human neural stem cells (hNSCs) transplanted in brain of mouse intracerebral hemorrhage (ICH) stroke model selectively migrate to the ICH lesion and induce behavioral recovery. However, low survival rate of grafted hNSCs in the brain precludes long-term therapeutic effect. We hypothesized that hNSCs overexpressing Akt1 transplanted into the lesion site could provide long-term improved survival of hNSCs, and behavioral recovery in mouse ICH model. F3 hNSC was genetically modified with a mouse Akt1 gene using a retroviral vector. F3 hNSCs expressing Akt1 were found to be highly resistant to H(2)O(2)-induced cytotoxicity in vitro. Following transplantation in ICH mouse brain, F3.Akt1 hNSCs induced behavioral improvement and significantly increased cell survival (50-100% increase) at 2 and 8 weeks post-transplantation as compared to parental F3 hNSCs. Brain transplantation of hNSCs overexpressing Akt1 in ICH animals provided functional recovery, and survival and differentiation of grafted hNSCs. These results indicate that the F3.Akt1 human NSCs should be a great value as a cellular source for the cellular therapy in animal models of human neurological disorders including ICH.
Conflict of interest statement
Figures




References
-
- Gebel JM, Broderick JP. Intracerebral hemorrhage. Neurol Clin. 2000;18:419–438. - PubMed
-
- NINDS ICH Workshop Participants. Priorities for clinical research in intracerebral hemorrhage: report from a National Institute of Neurological Disorders and Stroke workshop. Stroke. 2005;36:23–41. - PubMed
-
- McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. - PubMed
-
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. - PubMed
-
- Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology. 2004;24:159–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

