p21 is required for dextrose-mediated inhibition of mouse liver regeneration
- PMID: 19441104
- PMCID: PMC2705473
- DOI: 10.1002/hep.22979
p21 is required for dextrose-mediated inhibition of mouse liver regeneration
Abstract
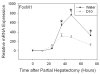
The inhibitory effect of dextrose supplementation on liver regeneration was first described more than 4 decades ago. Nevertheless, the molecular mechanisms responsible for this observation have not been elucidated. We investigated these mechanisms using the partial hepatectomy model in mice given standard or 10% dextrose (D10)-supplemented drinking water. The results showed that D10-treated mice exhibited significantly reduced hepatic regeneration compared with controls, as assessed by hepatocellular bromodeoxyuridine (BrdU) incorporation and mitotic frequency. D10 supplementation did not suppress activation of hepatocyte growth factor (HGF), induction of transforming growth factor alpha (TGF-alpha) expression, or tumor necrosis factor alpha-interleukin-6 cytokine signaling, p42/44 extracellular signal-regulated kinase (ERK) activation, immediate early gene expression, or expression of CCAAT/enhancer binding protein beta (C/EBPbeta), but did augment expression of the mito-inhibitory factors C/EBPalpha, p21(Waf1/Cip1), and p27(Kip1). In addition, forkhead box M1 (FoxM1) expression, which is required for normal liver regeneration, was suppressed by D10 treatment. Finally, D10 did not suppress either FoxM1 expression or hepatocellular proliferation in p21 null mice subjected to partial hepatectomy, establishing the functional significance of these events in mediating the effects of D10 on liver regeneration.
Conclusion: These data show that the inhibitory effect of dextrose supplementation on liver regeneration is associated with increased expression of C/EBPalpha, p21, and p27, and decreased expression of FoxM1, and that D10-mediated inhibition of liver regeneration is abrogated in p21-deficient animals. Our observations are consistent with a model in which hepatic sufficiency is defined by homeostasis between the energy-generating capacity of the liver and the energy demands of the body mass, with liver regeneration initiated when the functional liver mass is no longer sufficient to meet such demand.
Figures







References
-
- Fausto N. Liver regeneration. J Hepatol. 2000;32(1 Suppl):19–31. - PubMed
-
- Diehl AM, Rai R. Liver Regeneration. In: Schiff R, Sorrell MF, Maddrey WC, editors. Schiff’s Diseases of the Liver. Philadelphia: Lippincott-Raven; 1999. pp. 39–54.
-
- Higgins GM, Anderson RM. Experimental Pathology of the Liver. 1. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
