Biomonitoring of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and its carcinogenic metabolites in urine
- PMID: 19441775
- PMCID: PMC3215276
- DOI: 10.1021/tx900052c
Biomonitoring of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and its carcinogenic metabolites in urine
Abstract
2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) is a carcinogenic heterocyclic aromatic amine that is produced in cooked meats. The simultaneous analysis of PhIP and its metabolites in human urine is a challenge, because these biomarkers only occur in urine at parts per billion or lower concentrations and must be selectively purifed from thousands of other urinary constituents. We have developed a facile solid-phase extraction method, employing a mixed-mode reverse-phase cation exchange resin, to simultaneously isolate PhIP, its glucuronide conjugates, and the glucuronide conjugates of the genotoxic metabolite 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine from the urine of meat eaters. PhIP and its metabolites were quantified by liquid chromatography-electrospray ionization/tandem mass spectrometry (LC-ESI/MS/MS), using a triple stage quadrupole mass spectrometer in the selected reaction monitoring scan mode. The lower limit of quantification (LOQ) of PhIP is 5 parts per trillion (ppt), and the LOQ values for the glucuronide conjugates are 50 ppt, when 25 microL of urine is employed for assay. The extraction scheme is versatile and has been employed to isolate other ring-hydroxylated and glucuronidated metabolites of PhIP, for characterization by LC-ESI/MS/MS.
Figures
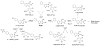




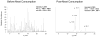


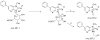

Similar articles
-
A comprehensive approach to the profiling of the cooked meat carcinogens 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, and their metabolites in human urine.Chem Res Toxicol. 2010 Apr 19;23(4):788-801. doi: 10.1021/tx900436m. Chem Res Toxicol. 2010. PMID: 20192249 Free PMC article.
-
Novel LC-ESI/MS/MS(n) method for the characterization and quantification of 2'-deoxyguanosine adducts of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by 2-D linear quadrupole ion trap mass spectrometry.Chem Res Toxicol. 2007 Feb;20(2):263-76. doi: 10.1021/tx0601713. Chem Res Toxicol. 2007. PMID: 17305409 Free PMC article.
-
Mapping serum albumin adducts of the food-borne carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by data-dependent tandem mass spectrometry.Chem Res Toxicol. 2012 Oct 15;25(10):2179-93. doi: 10.1021/tx300253j. Epub 2012 Aug 14. Chem Res Toxicol. 2012. PMID: 22827630 Free PMC article.
-
Metabolites of 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine (PhIP) in human urine after consumption of charbroiled or fried beef.Mutat Res. 2002 Sep 30;506-507:163-73. doi: 10.1016/s0027-5107(02)00163-x. Mutat Res. 2002. PMID: 12351156 Review.
-
Metabolism of the food carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in the rat and other rodents.Princess Takamatsu Symp. 1995;23:113-22. Princess Takamatsu Symp. 1995. PMID: 8844802 Review.
Cited by
-
The food-borne carcinogenic 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) disrupts circadian rhythms and ameliorated by pterostilbene (PSB) in Caenorhabditis elegans.Arch Toxicol. 2024 Dec;98(12):4131-4141. doi: 10.1007/s00204-024-03857-5. Epub 2024 Sep 10. Arch Toxicol. 2024. PMID: 39254834
-
Effect of Cytochrome P450 Reductase Deficiency on 2-Amino-9H-pyrido[2,3-b]indole Metabolism and DNA Adduct Formation in Liver and Extrahepatic Tissues of Mice.Chem Res Toxicol. 2015 Dec 21;28(12):2400-10. doi: 10.1021/acs.chemrestox.5b00405. Epub 2015 Dec 3. Chem Res Toxicol. 2015. PMID: 26583703 Free PMC article.
-
Mass Spectrometric Characterization of Human Serum Albumin Adducts Formed with N-Oxidized Metabolites of 2-Amino-1-methylphenylimidazo[4,5-b]pyridine in Human Plasma and Hepatocytes.Chem Res Toxicol. 2015 May 18;28(5):1045-59. doi: 10.1021/acs.chemrestox.5b00075. Epub 2015 Apr 10. Chem Res Toxicol. 2015. PMID: 25815793 Free PMC article.
-
Biomonitoring the cooked meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in hair: impact of exposure, hair pigmentation, and cytochrome P450 1A2 phenotype.Cancer Epidemiol Biomarkers Prev. 2013 Mar;22(3):356-64. doi: 10.1158/1055-9965.EPI-12-1206. Epub 2013 Jan 17. Cancer Epidemiol Biomarkers Prev. 2013. PMID: 23329727 Free PMC article.
-
A comprehensive approach to the profiling of the cooked meat carcinogens 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, and their metabolites in human urine.Chem Res Toxicol. 2010 Apr 19;23(4):788-801. doi: 10.1021/tx900436m. Chem Res Toxicol. 2010. PMID: 20192249 Free PMC article.
References
-
- Felton JS, Knize MG, Bennett LM, Malfatti MA, Colvin ME, Kulp KS. Impact of environmental exposures on the mutagenicity/carcinogenicity of heterocyclic amines. Toxicology. 2004;198:135–145. - PubMed
-
- Ni W, McNaughton L, LeMaster DM, Sinha R, Turesky RJ. Quantitation of 13 heterocyclic aromatic amines in cooked beef, pork, and chicken by liquid chromatography-electrospray iIonization/tandem mass spectrometry. J Agric Food Chem. 2008;56:68–78. - PubMed
-
- Friesen MD, Kaderlik K, Lin D, Garren L, Bartsch H, Lang NP, Kadlubar FF. Analysis of DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in rat and human tissues by alkaline hydrolysis and gas chromatography/electron capture mass spectrometry: validation by comparison with 32P-postlabeling. Chem Res Toxicol. 1994;7:733–739. - PubMed
-
- Magagnotti C, Pastorelli R, Pozzi S, Andreoni B, Fanelli R, Airoldi L. Genetic polymorphisms and modulation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-DNA adducts in human lymphocytes. Int J Cancer. 2003;107:878–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

