Degraded protein adducts of cis-2-butene-1,4-dial are urinary and hepatocyte metabolites of furan
- PMID: 19441776
- PMCID: PMC2696637
- DOI: 10.1021/tx800377v
Degraded protein adducts of cis-2-butene-1,4-dial are urinary and hepatocyte metabolites of furan
Abstract
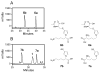
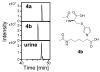
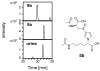
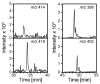
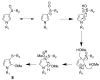
Furan is a liver toxicant and carcinogen in rodents. On the basis of these observations and the large potential for human exposure, furan has been classified as a possible human carcinogen. The mechanism of tumor induction by furan is unknown. However, the toxicity requires cytochrome P450-catalyzed oxidation of furan. The product of this oxidation, cis-2-butene-1,4-dial (BDA), reacts readily with glutathione, amino acids, and DNA and is a bacterial mutagen in Ames assay strain TA104. Characterization of the urinary metabolites of furan is expected to provide information regarding the structure(s) of the reactive metabolite(s). Recently, several urinary metabolites have been identified. We reported the presence of a monoglutathione-BDA reaction product, N-[4-carboxy-4-(3-mercapto-1H-pyrrol-1-yl)-1-oxobutyl]-l-cysteinylglycine cyclic sulfide. Three additional urinary metabolites of furan were also characterized as follows: R-2-acetylamino-6-(2,5-dihydro-2-oxo-1H-pyrrol-1-yl)-1-hexanoic acid, N-acetyl-S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]-l-cysteine, and its sulfoxide. It was postulated that these three metabolites are derived from degraded protein adducts. However, the possibility that these metabolites result from the reaction of BDA with free lysine and/or cysteine was not ruled out. In this latter case, one might predict that the reaction of thiol-BDA with free lysine would not occur exclusively on the epsilon-amino group. Reaction of BDA with N-acetylcysteine or GSH in the presence of lysine indicated that both the alpha- and the epsilon-amino groups of lysine can be modified by thiol-BDA. The N-acetylcysteine-BDA-N-acetyllysine urinary metabolites were solely linked through the epsilon-amino group of lysine. A GSH-BDA-lysine cross-link was a significant hepatocyte metabolite of furan. In this case, the major product resulted from reaction with the epsilon-amino group of lysine; however, small amounts of the alpha-amino reaction product were also observed. Western analysis of liver and hepatocyte protein extracts using anti-GSH antibody indicated that GSH was covalently linked to proteins in tissues or cells exposed to furan. Our data support the hypothesis that GSH-BDA can react with either free lysine or protein lysine groups. These data suggest that there are multiple pathways by which furan can modify cellular nucleophiles. In one pathway, BDA reacts directly with proteins to form cysteine-lysine reaction products. In another, BDA reacts with GSH to form GSH-BDA conjugates, which then react with cellular nucleophiles like free lysine or lysine moieties in proteins. Both pathways will give rise to N-acetyl-S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]-l-cysteine. Given the abundance of these metabolites in urine of furan-treated rats, these pathways appear to be major pathways of furan biotransformation in vivo.
Figures




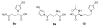
References
-
- International Agency for Research on Cancer. Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals. Vol. 53. IARC; Lyon: 1995. Furan; p. 393.
-
- Maga J. Furans in foods. Crit Rev Food Sci Nutr. 1979;11:355–366. - PubMed
-
- Capurro PU. Effects of exposure to solvents caused by air pollution with special reference to CCl4 and its distribution in air. Clin Toxicol. 1973;6:109–124. - PubMed
-
- Crews C, Castle L. A review of the occurrence, formation and analysis of furan in heat-processed foods. Trends Food Sci Technol. 2007;18:365–372.
-
- National Toxicology Program. 11th Report on Carcinogens. US Department of Health and Human Services; Washington DC: 2005.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

