Negishi coupling of secondary alkylzinc halides with aryl bromides and chlorides
- PMID: 19441851
- PMCID: PMC2746668
- DOI: 10.1021/ja902046m
Negishi coupling of secondary alkylzinc halides with aryl bromides and chlorides
Abstract
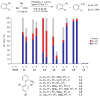
An efficient palladium-catalyzed process has been developed for Negishi coupling of secondary alkylzinc halides with a wide range of aryl bromides and activated aryl chlorides. A palladium catalyst composed of a new biaryldialkylphosphine ligand, CPhos, effectively promotes the rate of the reductive elimination step relative to the rate of the undesired beta-hydride elimination. The broad substrate scope and excellent ratio of the desired secondary to the undesired primary coupling product make this method a powerful and reliable tool for C(sp(3))-C(sp(2)) bond formation.
Figures

Similar articles
-
Simple, efficient catalyst system for the palladium-catalyzed amination of aryl chlorides, bromides, and triflates.J Org Chem. 2000 Feb 25;65(4):1158-74. doi: 10.1021/jo991699y. J Org Chem. 2000. PMID: 10814067
-
Pd-PEPPSI-IPent(Cl): a highly effective catalyst for the selective cross-coupling of secondary organozinc reagents.Angew Chem Int Ed Engl. 2012 Nov 5;51(45):11354-7. doi: 10.1002/anie.201205747. Epub 2012 Oct 4. Angew Chem Int Ed Engl. 2012. PMID: 23038603
-
AlAr3(THF): highly efficient reagents for cross-couplings with aryl bromides and chlorides catalyzed by the economic palladium complex of PCy3.Chem Commun (Camb). 2007 Oct 7;(37):3847-9. doi: 10.1039/b707703c. Chem Commun (Camb). 2007. PMID: 18217667
-
Palladium-catalyzed negishi cross-coupling reactions of unactivated alkyl iodides, bromides, chlorides, and tosylates.J Am Chem Soc. 2003 Oct 15;125(41):12527-30. doi: 10.1021/ja0363258. J Am Chem Soc. 2003. PMID: 14531697
-
A general and efficient catalyst for palladium-catalyzed C-O coupling reactions of aryl halides with primary alcohols.J Am Chem Soc. 2010 Aug 25;132(33):11592-8. doi: 10.1021/ja103248d. J Am Chem Soc. 2010. PMID: 20672810
Cited by
-
Organozinc Chemistry Enabled by Micellar Catalysis. Palladium-Catalyzed Cross-Couplings between Alkyl and Aryl Bromides in Water at Room Temperature.Organometallics. 2011 Nov 28;30(22):6090-6097. doi: 10.1021/om200846h. Epub 2011 Nov 21. Organometallics. 2011. PMID: 23539206 Free PMC article.
-
Original 2-(3-Alkoxy-1H-pyrazol-1-yl)azines Inhibitors of Human Dihydroorotate Dehydrogenase (DHODH).J Med Chem. 2015 Jul 23;58(14):5579-98. doi: 10.1021/acs.jmedchem.5b00606. Epub 2015 Jun 30. J Med Chem. 2015. PMID: 26079043 Free PMC article.
-
Selective ortho-Functionalization of Adamantylarenes Enabled by Dispersion and an Air-Stable Palladium(I) Dimer.Angew Chem Int Ed Engl. 2020 May 11;59(20):7721-7725. doi: 10.1002/anie.202001326. Epub 2020 Mar 17. Angew Chem Int Ed Engl. 2020. PMID: 32065717 Free PMC article.
-
Cross-Coupling of Gaseous Alkanes with (Hetero)Aryl Bromides via Dual Nickel/Photoredox Catalysis.Angew Chem Int Ed Engl. 2025 Jan 10;64(2):e202416957. doi: 10.1002/anie.202416957. Epub 2024 Nov 2. Angew Chem Int Ed Engl. 2025. PMID: 39316730 Free PMC article.
-
C-C bond formation via copper-catalyzed conjugate addition reactions to enones in water at room temperature.J Am Chem Soc. 2012 Dec 12;134(49):19985-8. doi: 10.1021/ja309409e. Epub 2012 Nov 28. J Am Chem Soc. 2012. PMID: 23190029 Free PMC article.
References
-
-
For recent reviews, see: de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. Wiley-VCH; New York: 2004. Tamao K, Miyaura N. Top Curr Chem. 2002;219:1.Hassan J, Sevignon M, Gozzi C, Schulz E, Lemaire M. Chem Rev. 2002;102:1359.
-
-
-
For representative references, see: Negishi coupling: Boudier A, Knochel P. Tetrahedron Lett. 1999;40:687.Dai C, Fu GC. J Am Chem Soc. 2001;123:2719.Corley EG, Conrad K, Murry JA, Savarin C, Holko J, Boice G. J Org Chem. 2004;69:5120.Kondolff I, Doucet H, Santelli M. Organometallics. 2006;25:5219.Luo X, Zhang H, Duan H, Liu Q, Zhu L, Zhang T, Lei A. Org Lett. 2007;9:4571.Melzig L, Gavryushin A, Knochel P. Org Lett. 2007;9:5529. Suzuki-Miyaura coupling: Dreher SD, Dormer PG, Sandrock DL, Molander GA. J Am Chem Soc. 2008;130:9257. and references cited therein. Kumada coupling: Tamao K, Kiso Y, Sumitani K, Kumada M. J Am Chem Soc. 1972;94:9268.Hayashi T, Konishi M, Kobori Y, Kumada M, Higuchi T, Hirotsu K. J Am Chem Soc. 1984;106:158.
-
-
-
For aryl-aryl Negishi coupling using RuPhos, see: Milne JE, Buchwald SL. J Am Chem Soc. 2004;126:13028. For Negishi coupling of aryl halides with primary alkyl zinc halides using SPhos, see: Manolikakes G, Schade MA, Hernandez CM, Mayr H, Knochel P. Org Lett. 2008;10:2765.Manolikakes G, Hernandez CM, Schade MA, Metzger A, Knochel P. J Org Chem. 2008;73:8422.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

