Diagnosing norovirus-associated infectious intestinal disease using viral load
- PMID: 19442278
- PMCID: PMC2698835
- DOI: 10.1186/1471-2334-9-63
Diagnosing norovirus-associated infectious intestinal disease using viral load
Abstract
Background: Reverse transcription-polymerase chain reaction (RT-PCR) is the main method for laboratory diagnosis of norovirus-associated infectious intestinal disease (IID). However, up to 16% of healthy individuals in the community, with no recent history of IID, may be RT-PCR positive; so it is unclear whether norovirus is actually the cause of illness in an IID case when they are RT-PCR positive. It is important to identify the pathogen causing illness in sporadic IID cases, for clinical management and for community based incidence studies. The aim of this study was to investigate how faecal viral load can be used to determine when norovirus is the most likely cause of illness in an IID case.
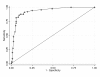
Methods: Real-time RT-PCR was used to determine the viral load in faecal specimens collected from 589 IID cases and 159 healthy controls, who were infected with genogroup II noroviruses. Cycle threshold (Ct) values from the real-time RT-PCR were used as a proxy measure of viral load. Receiver-operating characteristic (ROC) analysis was used to identify a cut-off in viral load for attributing illness to norovirus in IID cases.
Results: One hundred and sixty-nine IID cases and 159 controls met the inclusion criteria for the ROC analysis. The optimal Ct value cut-off for attributing IID to norovirus was 31. The same cut-off was selected when using healthy controls, or IID cases who were positive by culture for bacterial pathogens, as the reference negative group. This alternative reference negative group can be identified amongst specimens routinely received in clinical virology laboratories.
Conclusion: We demonstrated that ROC analysis can be used to select a cut-off for a norovirus real time RT-PCR assay, to aid clinical interpretation and diagnose when norovirus is the cause of IID. Specimens routinely received for diagnosis in clinical virology laboratories can be used to select an appropriate cut-off. Individual laboratories can use this method to define in-house cut-offs for their assays, to provide the best possible diagnostic service to clinicians and public health workers. Other clinical and epidemiological information should also be considered for patients with Ct values close to the cut-off, for the most accurate diagnosis of IID aetiology.
Figures


References
-
- Houde A, Leblanc D, Poitras E, Ward P, Brassard J, Simard C. Comparative evaluation of RT-PCR, nucleic acid sequence-based amplification (NASBA) and real-time RT-PCR for detection of noroviruses in faecal material. J Virol Methods. 2006;135:163–172. doi: 10.1016/j.jviromet.2006.03.001. - DOI - PubMed
-
- Medici MC, Martinelli M, Ruggeri FM, Abelli LA, Bosco S, Arcangeletti MC. Broadly reactive nested reverse transcription-PCR using an internal RNA standard control for detection of noroviruses in stool samples. J Clin Microbiol. 2005;43:3772–3778. doi: 10.1128/JCM.43.8.3772-3778.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

