Distinct pathways of desensitization of A1- and A2-adenosine receptors in DDT1 MF-2 cells
- PMID: 1944235
- PMCID: PMC5602552
Distinct pathways of desensitization of A1- and A2-adenosine receptors in DDT1 MF-2 cells
Abstract
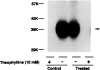
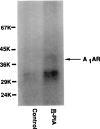
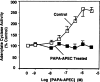
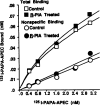
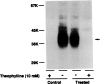
Desensitization of adenosine receptors (ARs) was studied in DDT1 MF-2 cells, which possess both A1- and A2AR, differentially coupled to adenylate cyclase. (-)-N6-(R)-Phenylisopropyladenosine (R-PIA), an A1AR-selective agonist at the appropriate concentrations, desensitized A1AR-mediated inhibition of adenylate cyclase activity in a time- (t1/2, 8 hr) and dose-dependent and reversible fashion. This was associated with significant decreases in total A1AR number and in the number of receptors possessing a high affinity for agonist in membrane preparations. The decrease in total A1AR in the membranes from the desensitized cells (approximately 40%) was associated with a 37% increase in A1AR measured in light vesicle preparations, compared with control cells. To test a possible role of phosphorylation in A1AR desensitization, cells were incubated with [32P]orthophosphate, followed by exposure to R-PIA for 18 hr. Subsequent purification of the A1AR indicated a 3-4-fold increase in phosphorylation of A1AR in cells treated with R-PIA, compared with control cells. Desensitization of the A1AR did not alter the levels of alpha s and alpha 12 proteins or affect the ability of stimulatory effectors, such as isoproterenol, sodium fluoride, and forskolin, to activate adenylate cyclase. These results suggest that uncoupling, down-regulation, and phosphorylation of the A1AR contribute, at least in part, to desensitization of this inhibitory receptor. Desensitization of the A2AR was characterized using an A2-selective agonist, 2-[4-(2-(4-aminophenyl]methylcarbonyl)ethyl)phenyl]ethylamino- 5'-N-ethylcarboxamidoadenosine (PAPA-APEC). Pretreatment of cells with PAPA-APEC (100 nM) resulted in a rapid loss of agonist stimulation of adenylate cyclase activity (t1/2 of this effect, 45 min). This effect was dose dependent (EC50, approximately 10 nM) and rapidly reversible. Interestingly, desensitization of the A2AR resulted in no change in receptor number, affinity, or mobility on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Taken together, these data suggest distinct mechanisms of desensitization of A1- and A2ARs in a single cell type.
Figures

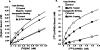
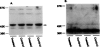

References
-
- Van Calker D, Muller M, Hamprecht B. Adenosine regulates via two different receptors the accumulation of cyclic AMP in cultured brain cells. J. Neurochem. 1979;33:999–1005. - PubMed
-
- Stiles GL. Adenosine receptors and beyond: molecular mechanisms of physiological regulation. Clin. Res. 1990;38:10–18. - PubMed
-
- Sperelakis N. Regulation of calcium slow channels of cardiac and smooth muscles by adenine nucleotides. In: Pelleg A, Michelson EL, Freifus LS, editors. Cardiac Electrophysiology and Pharmacology of Adenosine and ATP: Basic and Clinical Aspects. Alan R. Liss, Inc.; New York: 1987. pp. 135–193. - PubMed
-
- Kurachi Y, Nakajima T, Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pfluegers Arch. 1986;407:264–274. - PubMed
-
- DeMazancourt P, Guidicelli Y. Guanine nucleotides and adenosine “Ri”-site analogues stimulate the membrane bound low Km cyclic AMP phosphodiesterase of rat adipocytes. FEBS Lett. 1984;173:385–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
