Joint loading-driven bone formation and signaling pathways predicted from genome-wide expression profiles
- PMID: 19442616
- PMCID: PMC2700035
- DOI: 10.1016/j.bone.2009.01.367
Joint loading-driven bone formation and signaling pathways predicted from genome-wide expression profiles
Abstract
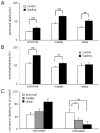
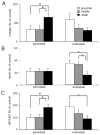
Joint loading is a recently developed loading modality that induces anabolic responses by lateral loads applied to a synovial joint such as an elbow and a knee. The present study extended this loading modality to an ankle and addressed a question: does ankle loading promote bone formation in the tibia? If so, what signaling pathways are involved in the anabolic responses? Using C57BL/6 female mice as a model system, lateral loads of 0.5 N were applied to the ankle at 5 Hz for 3 min/day for 3 consecutive days and load-driven bone formation was evaluated at three tibial cross-sections (the proximal, middle, and distal diaphysis). Furthermore, total RNA was isolated for 3 pairs of microarray experiments as well as quantitative real-time PCR analyses. The histomorphometric results revealed that in all cross-sections ankle loading elevated the cortical area and thickness as well as the calcein-labeled surface. Signaling pathway analysis from microarray-derived whole-genome mRNA expression profiles and quantitative real-time PCR predicted that molecules in phosphoinositide 3-kinase (PI3K), ECM-receptor interactions, TGFbeta signaling, and Wnt signaling were involved in the joint-loading driven responses. Since ankle loading stimulates bone formation throughout the tibia both in the endosteum and the periosteum, it may provide a non-pharmacological approach to effectively activate molecular signaling necessary for preventing bone loss.
Figures






Similar articles
-
Osteogenic potentials with joint-loading modality.J Bone Miner Metab. 2005;23(4):302-8. doi: 10.1007/s00774-005-0603-x. J Bone Miner Metab. 2005. PMID: 15981026
-
Knee loading stimulates cortical bone formation in murine femurs.BMC Musculoskelet Disord. 2006 Sep 19;7:73. doi: 10.1186/1471-2474-7-73. BMC Musculoskelet Disord. 2006. PMID: 16984642 Free PMC article.
-
Diaphyseal bone formation in murine tibiae in response to knee loading.J Appl Physiol (1985). 2006 May;100(5):1452-9. doi: 10.1152/japplphysiol.00997.2005. Epub 2006 Jan 12. J Appl Physiol (1985). 2006. PMID: 16410382
-
Joint loading modality: its application to bone formation and fracture healing.Br J Sports Med. 2008 Jul;42(7):556-60. doi: 10.1136/bjsm.2007.042556. Epub 2007 Nov 29. Br J Sports Med. 2008. PMID: 18048437 Free PMC article. Review.
-
Biomolecular Cell-Signaling Mechanisms and Dental Implants: A Review on the Regulatory Molecular Biologic Patterns Under Functional and Immediate Loading.Int J Oral Maxillofac Implants. 2016 Jul-Aug;31(4):939-51. doi: 10.11607/jomi.4384. Int J Oral Maxillofac Implants. 2016. PMID: 27447163 Review.
Cited by
-
Lengthening of mouse hindlimbs with joint loading.J Bone Miner Metab. 2010 May;28(3):268-75. doi: 10.1007/s00774-009-0135-x. Epub 2009 Nov 5. J Bone Miner Metab. 2010. PMID: 19890688
-
Molecular genetic studies of gene identification for osteoporosis: the 2009 update.Endocr Rev. 2010 Aug;31(4):447-505. doi: 10.1210/er.2009-0032. Epub 2010 Mar 31. Endocr Rev. 2010. PMID: 20357209 Free PMC article. Review.
-
Mechanical loading: bone remodeling and cartilage maintenance.Curr Osteoporos Rep. 2011 Dec;9(4):237-42. doi: 10.1007/s11914-011-0067-y. Curr Osteoporos Rep. 2011. PMID: 21858507 Review.
-
CRLF1 and CLCF1 in Development, Health and Disease.Int J Mol Sci. 2022 Jan 17;23(2):992. doi: 10.3390/ijms23020992. Int J Mol Sci. 2022. PMID: 35055176 Free PMC article. Review.
-
Knee loading protects against osteonecrosis of the femoral head by enhancing vessel remodeling and bone healing.Bone. 2015 Dec;81:620-631. doi: 10.1016/j.bone.2015.09.012. Epub 2015 Sep 28. Bone. 2015. PMID: 26416150 Free PMC article.
References
-
- Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone. 2002;30:781–6. - PubMed
-
- Rubin C, Turner AS, Muller R, Mittra E, McLeod K, Lin W, et al. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17:349–57. - PubMed
-
- Flieger J, Karachalios T, Khaldi L, Raptou P, Lyritis G. Mechanical stimulation in the form of vibration prevents postmenopausal bone loss in ovariectomized rats. Calcif Tissue Int. 1998;63:510–4. - PubMed
-
- Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low Mechanical signals strengthen long bones. Nature. 201(412):603–4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

