Neurosteroid production in the songbird brain: a re-evaluation of core principles
- PMID: 19442685
- PMCID: PMC2724309
- DOI: 10.1016/j.yfrne.2009.05.001
Neurosteroid production in the songbird brain: a re-evaluation of core principles
Abstract
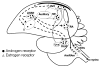
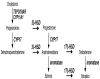
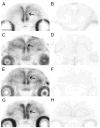
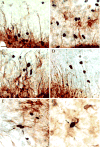
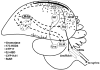
Concepts of brain-steroid signaling have traditionally placed emphasis on the gonads and adrenals as the source of steroids, the strict dichotomy of early developmental ("organizational") and mature ("activational") effects, and a relatively slow mechanism of signaling through intranuclear receptors. Continuing research shows that these concepts are not inaccurate, but they are certainly incomplete. In this review, we focus on the song control circuit of songbird species to demonstrate how each of these concepts is limited. We discuss the solid evidence for steroid synthesis within the brain ("neurosteroidogenesis"), the role of neurosteroids in organizational events that occur both early in development and later in life, and how neurosteroids can act in acute and non-traditional ways. The songbird model therefore illustrates how neurosteroids can dramatically increase the diversity of steroid-sensitive brain functions in a behaviorally-relevant system. We hope this inspires further research and thought into neurosteroid signaling in songbirds and other animals.
Figures
Similar articles
-
Neurosteroidogenesis: insights from studies of songbirds.J Neuroendocrinol. 2012 Jan;24(1):16-21. doi: 10.1111/j.1365-2826.2011.02150.x. J Neuroendocrinol. 2012. PMID: 21535249 Free PMC article. Review.
-
Birdsong and the neural production of steroids.J Chem Neuroanat. 2010 Mar;39(2):72-81. doi: 10.1016/j.jchemneu.2009.06.009. Epub 2009 Jul 7. J Chem Neuroanat. 2010. PMID: 19589382 Free PMC article. Review.
-
Widespread capacity for steroid synthesis in the avian brain and song system.Endocrinology. 2006 Dec;147(12):5975-87. doi: 10.1210/en.2006-0154. Epub 2006 Aug 24. Endocrinology. 2006. PMID: 16935847 Free PMC article.
-
Neurosteroids and the songbird model system.J Exp Zool A Comp Exp Biol. 2006 Sep 1;305(9):743-8. doi: 10.1002/jez.a.303. J Exp Zool A Comp Exp Biol. 2006. PMID: 16902969 Review.
-
Neurosteroids: biosynthesis and function.Crit Rev Neurobiol. 1995;9(4):383-94. Crit Rev Neurobiol. 1995. PMID: 8829852 Review.
Cited by
-
Neural expression and post-transcriptional dosage compensation of the steroid metabolic enzyme 17beta-HSD type 4.BMC Neurosci. 2010 Apr 1;11:47. doi: 10.1186/1471-2202-11-47. BMC Neurosci. 2010. PMID: 20359329 Free PMC article.
-
Steroids in the Avian Brain: Heterogeneity across Space and Time.J Ornithol. 2015 Dec 1;156(Suppl 1):419-424. doi: 10.1007/s10336-015-1184-7. Epub 2015 Mar 13. J Ornithol. 2015. PMID: 26924851 Free PMC article.
-
The genome of a songbird.Nature. 2010 Apr 1;464(7289):757-62. doi: 10.1038/nature08819. Nature. 2010. PMID: 20360741 Free PMC article.
-
Synaptocrine signaling: steroid synthesis and action at the synapse.Endocr Rev. 2011 Aug;32(4):532-49. doi: 10.1210/er.2011-0004. Epub 2011 May 26. Endocr Rev. 2011. PMID: 21622487 Free PMC article. Review.
-
Estradiol-dependent modulation of auditory processing and selectivity in songbirds.Front Neuroendocrinol. 2011 Aug;32(3):287-302. doi: 10.1016/j.yfrne.2010.12.002. Epub 2010 Dec 10. Front Neuroendocrinol. 2011. PMID: 21146556 Free PMC article. Review.
References
-
- Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Ann Rev Neurosci. 1984;7:413–42. - PubMed
-
- Ball GF. Neuroendocrine basis of seasonal changes in vocal behavior among songbirds. In: Hauser M, Konishi M, editors. Neural Mechanisms of Communication. MIT Press; Cambridge, MA: 2000. pp. 213–253.
-
- Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: Multiple sites of action of sex steroid hormones. Frontiers in Neuroendocrinology. 2002;23:137–178. - PubMed
-
- Balthazart J, Ball GF. Sexual differentiation of brain and behavior in birds. Trends Endocrinol Metab. 1995;6:21–29. - PubMed
-
- Bottjer SW, Johnson F. Circuits, hormones, and learning: vocal behavior in songbirds. J Neurobiol. 1997;33:602–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

