Diadenosine tetraphosphate reduces toxicity caused by high-dose methamphetamine administration
- PMID: 19442829
- PMCID: PMC2759191
- DOI: 10.1016/j.neuro.2009.02.003
Diadenosine tetraphosphate reduces toxicity caused by high-dose methamphetamine administration
Abstract
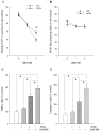
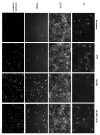
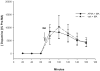
Diadenosine tetraphosphate (AP(4)A), two adenosine moieties bridged by four phosphates, is an endogenous purinergic ligand found in brain. Previous studies have shown that AP(4)A reduced neurodegeneration caused by the dopaminergic neurotoxin 6-hydroxydopamine in rat striatum and substantia nigra. The purpose of this study was to determine whether AP(4)A is protective against methamphetamine (MA)-mediated toxicity. Primary neuronal cultures were prepared from rat embryonic (E14-E15) ventral mesencephalic tissue. Cultures treated with 2mM MA exhibited decreased tyrosine hydroxylase (TH) immunoreactivity and increased cleaved caspase-3 immunoreactivity and TUNEL labeling. All these changes were lessened by pretreatment with AP(4)A. The protective effect of AP(4)A was also found in vivo. Adult Sprague-Dawley rats were injected with AP(4)A (25 microg/20 microl) or vehicle intracerebroventricularly followed by 4 doses of MA (5 or 10 mg/kg), given subcutaneously every 2h. Administration of MA reduced locomotor activity 1 day after injection, which was significantly antagonized by the pretreatment with AP(4)A. Using immunohistochemical analysis, TH fiber density at the substantia nigra pars reticulata was found reduced while cleaved caspase-3 immunoreactivity in striatum was increased after MA treatment; these responses were also significantly antagonized by AP(4)A. Taken together, our data show that AP(4)A has protective effects against MA-mediated toxicity both in vitro and in vivo. The mechanism of action involves suppression of MA-induced apoptosis.
Figures




Similar articles
-
9-Cis retinoic acid protects against methamphetamine-induced neurotoxicity in nigrostriatal dopamine neurons.Neurotox Res. 2014 Apr;25(3):248-61. doi: 10.1007/s12640-013-9413-4. Epub 2013 Jul 25. Neurotox Res. 2014. PMID: 23884514 Free PMC article.
-
Bone morphogenetic protein-7 reduces toxicity induced by high doses of methamphetamine in rodents.Neuroscience. 2008 Jan 2;151(1):92-103. doi: 10.1016/j.neuroscience.2007.10.044. Epub 2007 Nov 13. Neuroscience. 2008. PMID: 18082966 Free PMC article.
-
Diadenosine tetraphosphate protects against injuries induced by ischemia and 6-hydroxydopamine in rat brain.J Neurosci. 2003 Aug 27;23(21):7958-65. doi: 10.1523/JNEUROSCI.23-21-07958.2003. J Neurosci. 2003. PMID: 12944527 Free PMC article.
-
Overexpression of parkin in the rat nigrostriatal dopamine system protects against methamphetamine neurotoxicity.Exp Neurol. 2013 Sep;247:359-72. doi: 10.1016/j.expneurol.2013.01.001. Epub 2013 Jan 9. Exp Neurol. 2013. PMID: 23313192 Free PMC article.
-
Early post-treatment with 9-cis retinoic acid reduces neurodegeneration of dopaminergic neurons in a rat model of Parkinson's disease.BMC Neurosci. 2012 Oct 6;13:120. doi: 10.1186/1471-2202-13-120. BMC Neurosci. 2012. PMID: 23040108 Free PMC article.
Cited by
-
Environmental enrichment does not reduce the rewarding and neurotoxic effects of methamphetamine.Neurotox Res. 2011 Jan;19(1):172-82. doi: 10.1007/s12640-010-9158-2. Epub 2010 Feb 9. Neurotox Res. 2011. PMID: 20143198
-
Increased Ap4A levels and ecto-nucleotidase activity in glaucomatous mice retina.Purinergic Signal. 2018 Sep;14(3):259-270. doi: 10.1007/s11302-018-9612-9. Epub 2018 Jun 8. Purinergic Signal. 2018. PMID: 29948577 Free PMC article.
-
Enhanced neurodegeneration after a high dose of methamphetamine in adenosine A3 receptor null mutant mice.Neuroscience. 2011 Oct 27;194:170-80. doi: 10.1016/j.neuroscience.2011.08.013. Epub 2011 Aug 10. Neuroscience. 2011. PMID: 21867746 Free PMC article.
-
Diadenosine tetraphosphate (Ap4A) inhibits ATP-induced excitotoxicity: a neuroprotective strategy for traumatic spinal cord injury treatment.Purinergic Signal. 2017 Mar;13(1):75-87. doi: 10.1007/s11302-016-9541-4. Epub 2016 Oct 19. Purinergic Signal. 2017. PMID: 27761681 Free PMC article.
-
Methamphetamine alters reference gene expression in nigra and striatum of adult rat brain.Neurotoxicology. 2013 Dec;39:138-45. doi: 10.1016/j.neuro.2013.08.009. Epub 2013 Sep 14. Neurotoxicology. 2013. PMID: 24042092 Free PMC article.
References
-
- Ali SF, Newport GD, Slikker W., Jr Methamphetamine-induced dopaminergic toxicity in mice. Role of environmental temperature and pharmacological agents. Ann N Y Acad Sci. 1996;801:187–98. - PubMed
-
- Bochner BR, Lee PC, Wilson SW, Cutler CW, Ames BN. AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell. 1984;37:225–32. - PubMed
-
- Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther. 1992;260:817–24. - PubMed
-
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–80. - PubMed
-
- Cadet JL, Ordonez SV, Ordonez JV. Methamphetamine induces apoptosis in immortalized neural cells: protection by the proto-oncogene, bcl-2. Synapse. 1997;25:176–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

