Histone H2a mRNA interacts with Lin28 and contains a Lin28-dependent posttranscriptional regulatory element
- PMID: 19443445
- PMCID: PMC2715237
- DOI: 10.1093/nar/gkp372
Histone H2a mRNA interacts with Lin28 and contains a Lin28-dependent posttranscriptional regulatory element
Abstract
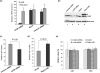
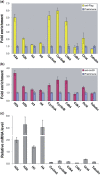
Lin28 has been shown to block the processing of let-7 microRNAs implicated in the regulation of cell growth and differentiation. Here, we show that Lin28 also specifically associates with ribonucleoprotein particles containing the replication-dependent histone H2a mRNA in mouse embryonic stem cells. We further show that the coding region of H2a mRNA harbors high affinity binding sequences for Lin28 and that these sequences stimulate the expression of reporter genes in a Lin28-dependent manner. We suggest that a key function of Lin28 in the maintenance of pluripotency is to promote the expression of the H2a gene (and perhaps also other replication-dependent histone genes) at the posttranscriptional level in order to coordinate histone production with the unique proliferative properties of embryonic stem cells.
Figures

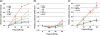

Similar articles
-
Lin28 modulates cell growth and associates with a subset of cell cycle regulator mRNAs in mouse embryonic stem cells.RNA. 2009 Mar;15(3):357-61. doi: 10.1261/rna.1368009. Epub 2009 Jan 15. RNA. 2009. PMID: 19147696 Free PMC article.
-
Lin28-mediated post-transcriptional regulation of Oct4 expression in human embryonic stem cells.Nucleic Acids Res. 2010 Mar;38(4):1240-8. doi: 10.1093/nar/gkp1071. Epub 2009 Dec 4. Nucleic Acids Res. 2010. PMID: 19966271 Free PMC article.
-
Lin28 is induced in primed embryonic stem cells and regulates let-7-independent events.FASEB J. 2017 Mar;31(3):1046-1058. doi: 10.1096/fj.201600848R. Epub 2016 Dec 5. FASEB J. 2017. PMID: 27920151
-
Embryonic stem cell microRNAs: defining factors in induced pluripotent (iPS) and cancer (CSC) stem cells?Curr Stem Cell Res Ther. 2009 Sep;4(3):168-77. doi: 10.2174/157488809789057400. Curr Stem Cell Res Ther. 2009. PMID: 19492978 Review.
-
Mechanisms of Lin28-mediated miRNA and mRNA regulation--a structural and functional perspective.Int J Mol Sci. 2013 Aug 9;14(8):16532-53. doi: 10.3390/ijms140816532. Int J Mol Sci. 2013. PMID: 23939427 Free PMC article. Review.
Cited by
-
An RNA chaperone, AtCSP2, negatively regulates salt stress tolerance.Plant Signal Behav. 2015;10(8):e1042637. doi: 10.1080/15592324.2015.1042637. Plant Signal Behav. 2015. PMID: 26252779 Free PMC article.
-
Determinants of mRNA recognition and translation regulation by Lin28.Nucleic Acids Res. 2012 Apr;40(8):3574-84. doi: 10.1093/nar/gkr1279. Epub 2011 Dec 30. Nucleic Acids Res. 2012. PMID: 22210884 Free PMC article.
-
Stage-Specific Timing of the microRNA Regulation of lin-28 by the Heterochronic Gene lin-14 in Caenorhabditis elegans.Genetics. 2017 Jan;205(1):251-262. doi: 10.1534/genetics.116.195040. Epub 2016 Nov 4. Genetics. 2017. PMID: 27815363 Free PMC article.
-
The Lin28 cold-shock domain remodels pre-let-7 microRNA.Nucleic Acids Res. 2012 Aug;40(15):7492-506. doi: 10.1093/nar/gks355. Epub 2012 May 8. Nucleic Acids Res. 2012. PMID: 22570413 Free PMC article.
-
Identification of mRNAs bound and regulated by human LIN28 proteins and molecular requirements for RNA recognition.RNA. 2013 May;19(5):613-26. doi: 10.1261/rna.036491.112. Epub 2013 Mar 12. RNA. 2013. PMID: 23481595 Free PMC article.
References
-
- Richard M, Tan SP, Tan JH, Chan WK, Bongso A. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells. 2004;22:51–64. - PubMed
-
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. - PubMed
-
- Rybak A, Fuchs H, Smirnova L, Brandt C, Pohl EE, Nitsch R, Wulczyn FG. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat. Cell Biol. 2008;10:987–993. - PubMed
-
- Piskounova E, Viswanathan SR, Janas M, Lapierre RJ, Daley GQ, Sliz P, Gregory RI. Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J. Biol. Chem. 2008;283:21310–21314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

