Kinetic and thermodynamic characterization of dihydrotestosterone-induced conformational perturbations in androgen receptor ligand-binding domain
- PMID: 19443608
- PMCID: PMC2718745
- DOI: 10.1210/me.2008-0304
Kinetic and thermodynamic characterization of dihydrotestosterone-induced conformational perturbations in androgen receptor ligand-binding domain
Abstract
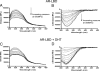
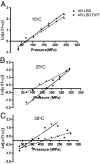
Ligand-induced conformational perturbations in androgen receptor (AR) are important in coactivator recruitment and transactivation. However, molecular rearrangements in AR ligand-binding domain (AR-LBD) associated with agonist binding and their kinetic and thermodynamic parameters are poorly understood. We used steady-state second-derivative absorption and emission spectroscopy, pressure and temperature perturbations, and 4,4'-bis-anilinonaphthalene 8-sulfonate (bis-ANS) partitioning to determine the kinetics and thermodynamics of the conformational changes in AR-LBD after dihydrotestosterone (DHT) binding. In presence of DHT, the second-derivative absorption spectrum showed a red shift and a change in peak-to-peak distance. Emission intensity increased upon DHT binding, and center of spectral mass was blue shifted, denoting conformational changes resulting in more hydrophobic environment for tyrosines and tryptophans within a more compact DHT-bound receptor. In pressure perturbation calorimetry, DHT-induced energetic stabilization increased the Gibbs free energy of unfolding to 8.4 +/- 1.3 kcal/mol from 3.5 +/- 1.6 kcal/mol. Bis-ANS partitioning studies revealed that upon DHT binding, AR-LBD underwent biphasic rearrangement with a high activation energy (13.4 kcal/mol). An initial, molten globule-like burst phase (k approximately 30 sec(-1)) with greater solvent accessibility was followed by rearrangement (k approximately 0.01 sec(-1)), leading to a more compact conformation than apo-AR-LBD. Molecular simulations demonstrated unique sensitivity of tyrosine and tryptophan residues during pressure unfolding with rearrangement of residues in the coactivator recruitment surfaces distant from the ligand-binding pocket. In conclusion, DHT binding leads to energetic stabilization of AR-LBD domain and substantial rearrangement of residues distant from the ligand-binding pocket. DHT binding to AR-LBD involves biphasic receptor rearrangement including population of a molten globule-like intermediate state.
Figures







References
-
- Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS 1995 Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev [Erratum (1995) 16:546] 16:271–321 - PubMed
-
- Brinkmann AO, Klaasen P, Kuiper GG, van der Korput JA, Bolt J, de Boer W, Smit A, Faber PW, van Rooij HC, Geurts van Kessel A, Mulder E, Trapman J 1989 Structure and function of the androgen receptor. Urol Res 17:87–93 - PubMed
-
- Gobinet J, Poujol N, Sultan Ch 2002 Molecular action of androgens. Mol Cell Endocrinol 198:15–24 - PubMed
-
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM 2006 Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 91:1995–2010 - PubMed
-
- Bhasin S 2007 Testicular disorders. In: Kronenberg H, Polonsky KS, Larsen PR, Melmed S, eds. Williams’ textbook of endocrinology. 11th ed. New York: Elsevier Health Sciences
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

