The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature
- PMID: 19443644
- PMCID: PMC2723985
- DOI: 10.1681/ASN.2008060640
The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature
Abstract
CXC chemokine ligand 12 (CXCL12; stromal cell-derived factor 1) is a unique homeostatic chemokine that signals through its cognate receptor, CXCR4. CXCL12/CXCR4 signaling is essential for the formation of blood vessels in the gastrointestinal tract during development, but its contribution to renal development remains unclear. Here, we found that CXCL12-secreting stromal cells surround CXCR4-positive epithelial components of early nephrons and blood vessels in the embryonic kidney. In glomeruli, we observed CXCL12-secreting podocytes in close proximity to CXCR4-positive endothelial cells. Both CXCL12- and CXCR4-deficient kidneys exhibited identical phenotypes; there were no apparent abnormalities in early nephrogenesis or in differentiation of podocytes and tubules, but there was defective formation of blood vessels, including ballooning of the developing glomerular tuft and disorganized patterning of the renal vasculature. To clarify the relative importance of different cellular defects resulting from ablation of CXCL12 and CXCR4, we established endothelial cell-specific CXCR4-deficient mice, which recapitulated the renal phenotypes of conventional CXCR4-deficient mice. We conclude that CXCL12 secreted from stromal cells or podocytes acts on endothelial cells to regulate vascular development in the kidney. These findings suggest new potential therapeutic targets for remodeling the injured kidney.
Figures
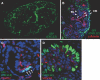

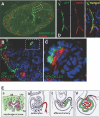
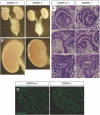
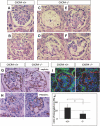
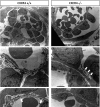
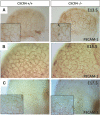

Comment in
-
The SDF-1/CXCR4 axis is a novel driver of vascular development of the glomerulus.J Am Soc Nephrol. 2009 Aug;20(8):1659-61. doi: 10.1681/ASN.2009060621. Epub 2009 Jul 16. J Am Soc Nephrol. 2009. PMID: 19608697 No abstract available.
References
-
- Vaughan MR, Quaggin SE: How do mesangial and endothelial cells form the glomerular tuft? J Am Soc Nephrol 19: 24–33, 2008 - PubMed
-
- Nagasawa T: A chemokine, SDF-1/PBSF, and its receptor, CXC chemokine receptor 4, as mediators of hematopoiesis. Int J Hematol 72: 408–411, 2000 - PubMed
-
- Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J: The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia 20: 1915–1924, 2006 - PubMed
-
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T: The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 393: 591–594, 1998 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

