Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network
- PMID: 19443659
- PMCID: PMC2713466
- DOI: 10.1182/blood-2009-03-212290
Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network
Abstract
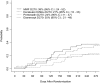
Acute graft-versus-host disease (aGVHD) is the primary limitation of allogeneic hematopoietic cell transplantation. Corticosteroids remain the standard initial therapy, yet only 25% to 41% of patients completely respond. This randomized, 4-arm, phase 2 trial was designed to identify the most promising agent(s) for initial therapy for aGVHD. Patients were randomized to receive methylprednisolone 2 mg/kg per day plus etanercept, mycophenolate mofetil (MMF), denileukin diftitox (denileukin), or pentostatin. Patients (n = 180) were randomized; their median age was 50 years (range, 7.5-70 years). Myeloablative conditioning represented 66% of transplants. Grafts were peripheral blood (61%), bone marrow (25%), or umbilical cord blood (14%); 53% were from unrelated donors. Patients who received MMF for prophylaxis (24%) were randomized to a non-MMF arm. At randomization, aGVHD was grade I to II (68%), III to IV (32%), and (53%) had visceral organ involvement. Day 28 complete response rates were etanercept 26%, MMF 60%, denileukin 53%, and pentostatin 38%. Corresponding 9-month overall survival was 47%, 64%, 49%, and 47%, respectively. Cumulative incidences of severe infections were as follows: etanercept 48%, MMF 44%, denileukin 62%, and pentostatin 57%. Efficacy and toxicity data suggest the use of MMF plus corticosteroids is the most promising regimen to compare against corticosteroids alone in a definitive phase 3 trial. This study is registered at http://www.clinicaltrials.gov as NCT00224874.
Figures
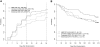


References
-
- Cragg L, Blazar BR, Defor T, et al. A randomized trial comparing prednisone with antithymocyte globulin/prednisone as an initial systemic therapy for moderately severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6:441–447. - PubMed
-
- Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–735. - PubMed
-
- Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. - PubMed
-
- Chao NJ, Schmidt GM, Niland JC, et al. Cyclosporine, methotrexate, and prednisone compared with cyclosporine and prednisone for prophylaxis of acute graft-versus-host disease. N Engl J Med. 1993;329:1225–1230. - PubMed
-
- Weisdorf D, Hakke R, Blazar B, et al. Risk factors for acute graft-versus-host disease in histocompatible donor bone marrow transplantation. Transplantation. 1991;51:1197–1203. - PubMed

