Neurotensin induces IL-6 secretion in mouse preadipocytes and adipose tissues during 2,4,6,-trinitrobenzensulphonic acid-induced colitis
- PMID: 19443690
- PMCID: PMC2688970
- DOI: 10.1073/pnas.0903499106
Neurotensin induces IL-6 secretion in mouse preadipocytes and adipose tissues during 2,4,6,-trinitrobenzensulphonic acid-induced colitis
Abstract
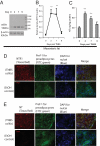
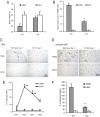
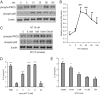
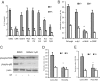
Mesenteric fat is known to undergo inflammatory changes after 2,4,6,-trinitrobenzensulphonic acid (TNBS)-induced colitis. Neurotensin (NT) and neurotensin receptor 1 (NTR1) have been shown to play a major role in the pathogenesis of intestinal inflammation. This led us to explore whether NT and NTR1 are expressed in the mesenteric fat depots during TNBS-induced colitis and whether NT participates in the increased interleukin (IL)-6 secretion in this inflammatory response. TNBS-induced inflammation in the colon increases NT and NTR1 expression in mesenteric adipose tissues, including mesenteric preadipocytes. Compared with wild-type mice, NT knockout (KO) mice have reduced TNBS-induced colitis accompanied by diminished inflammatory responses in mesenteric adipose tissue. Specifically, IL-6 and p65 phosphorylation levels in mesenteric fat of NT KO mice are also reduced compared with wild-type mice. Mouse 3T3-L1 preadipocytes express NTR1 and its expression is increased after stimulation of preadipocytes with proinflammatory cytokines. NT stimulation of 3T3-L1 preadipocytes overexpressing NTR1 causes PKCdelta phosphorylation and IL-6 secretion in a time- and dose-dependent fashion. Moreover, NT-mediated IL-6 expression is nuclear factor-kappaB and PKCdelta dependent. We also found that supernatants from NT-exposed 3T3-L1-NTR1 preadipocytes and mesenteric fat obtained from wild-type mice 2 days after TNBS administration stimulate an IL-6-dependent macrophage migration measured by a macrophage migration assay, whereas this response is reduced when mesenteric fat from NT KO mice is used. These results demonstrate an important role for NT in acute colitis and adipose tissue inflammation associated with experimental colitis that involves direct NT proinflammatory responses in preadipocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. - PubMed
-
- Desreumaux P, et al. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology. 1999;117:73–81. - PubMed
-
- Schaffler A, Scholmerich J, Buchler C. Mechanisms of disease: Adipocytokines and visceral adipose tissue—emerging role in nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:273–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

