Platelet-derived hyaluronidase 2 cleaves hyaluronan into fragments that trigger monocyte-mediated production of proinflammatory cytokines
- PMID: 19443707
- PMCID: PMC2684190
- DOI: 10.2353/ajpath.2009.080831
Platelet-derived hyaluronidase 2 cleaves hyaluronan into fragments that trigger monocyte-mediated production of proinflammatory cytokines
Abstract
Hyaluronan (HA) occurs in the body as a large, hydrating, space-filling, carbohydrate polymer in the extracellular matrix; it has both anti-angiogenic and immunosuppressive properties. Cleavage of HA results in the generation of variably sized fragments that stimulate multiple angiogenic and inflammatory responses in a size-specific manner. In this study, we report that platelets, as well as their megakaryocyte precursors, are unusual among somatic cells in that they contain only hyaluronidase 2 (HYAL2) but not HYAL1. Platelet HYAL2 is sufficient to cleave HA into fragments that are specific for inflammatory and angiogenic signaling; this process occurs in the absence of HYAL1, which is necessary in all other tissues to perform further HA degradation. Platelets can bind to HA, some of which derives from the stressed microvessel endothelial cell surface. Platelet-derived HYAL2 cleaves HA into fragments that stimulate mononuclear leukocytes in the immediate microenvironment to produce proinflammatory cytokines, including interleukin-6 and interleukin-8. Platelets, thus, are not only involved in hemostasis, the earliest step in wound healing, but are also important in the signaling of subsequent inflammatory and angiogenic steps. We hypothesize that aberrations in these sequential steps can promote chronic inflammation, as found in inflammatory bowel disease. The platelet may thus provide an interface between acute and chronic inflammation, wound healing, and their subsequent fibrotic responses.
Figures






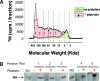
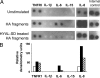

Comment in
-
Hyaluronan, platelets, and monocytes: a novel pro-inflammatory triad.Am J Pathol. 2009 Jun;174(6):1993-5. doi: 10.2353/ajpath.2009.081138. Epub 2009 May 12. Am J Pathol. 2009. PMID: 19435789 Free PMC article. Review.
Similar articles
-
Platelet hyaluronidase-2: an enzyme that translocates to the surface upon activation to function in extracellular matrix degradation.Blood. 2015 Feb 26;125(9):1460-9. doi: 10.1182/blood-2014-07-590513. Epub 2014 Nov 19. Blood. 2015. PMID: 25411425 Free PMC article.
-
Hyal2 Expression in Tumor-Associated Myeloid Cells Mediates Cancer-Related Inflammation in Bladder Cancer.Cancer Res. 2021 Feb 1;81(3):648-657. doi: 10.1158/0008-5472.CAN-20-1144. Epub 2020 Nov 25. Cancer Res. 2021. PMID: 33239427
-
Platelet hyaluronidase-2 regulates the early stages of inflammatory disease in colitis.Blood. 2019 Aug 29;134(9):765-775. doi: 10.1182/blood.2018893594. Epub 2019 Jul 1. Blood. 2019. PMID: 31262781 Free PMC article.
-
Endothelial Glycocalyx as a Shield Against Diabetic Vascular Complications: Involvement of Hyaluronan and Hyaluronidases.Arterioscler Thromb Vasc Biol. 2018 Jul;38(7):1427-1439. doi: 10.1161/ATVBAHA.118.310839. Epub 2018 Jun 7. Arterioscler Thromb Vasc Biol. 2018. PMID: 29880486 Free PMC article. Review.
-
Genetic Deficiencies of Hyaluronan Degradation.Cells. 2024 Jul 16;13(14):1203. doi: 10.3390/cells13141203. Cells. 2024. PMID: 39056785 Free PMC article. Review.
Cited by
-
A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds.Am J Pathol. 2012 Oct;181(4):1250-70. doi: 10.1016/j.ajpath.2012.06.036. Epub 2012 Aug 11. Am J Pathol. 2012. PMID: 22889846 Free PMC article.
-
Origin and Function of Monocytes in Inflammatory Bowel Disease.J Inflamm Res. 2024 May 13;17:2897-2914. doi: 10.2147/JIR.S450801. eCollection 2024. J Inflamm Res. 2024. PMID: 38764499 Free PMC article. Review.
-
Thrombin Cleavage of Inter-α-inhibitor Heavy Chain 1 Regulates Leukocyte Binding to an Inflammatory Hyaluronan Matrix.J Biol Chem. 2016 Nov 18;291(47):24324-24334. doi: 10.1074/jbc.M116.755660. Epub 2016 Sep 27. J Biol Chem. 2016. PMID: 27679489 Free PMC article.
-
Hyaluronan in intestinal homeostasis and inflammation: implications for fibrosis.Am J Physiol Gastrointest Liver Physiol. 2011 Dec;301(6):G945-9. doi: 10.1152/ajpgi.00063.2011. Epub 2011 Aug 18. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21852366 Free PMC article. Review.
-
Platelet hyaluronidase-2: an enzyme that translocates to the surface upon activation to function in extracellular matrix degradation.Blood. 2015 Feb 26;125(9):1460-9. doi: 10.1182/blood-2014-07-590513. Epub 2014 Nov 19. Blood. 2015. PMID: 25411425 Free PMC article.
References
-
- Lee JY, Spicer AP. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol. 2000;12:581–586. - PubMed
-
- Day AJ, Sheehan JK. Hyaluronan: polysaccharide chaos to protein organization. Curr Opin Struct Biol. 2001;11:617–622. - PubMed
-
- Feinberg RN, Beebe DC. Hyaluronate in vasculogenesis. Science. 1983;220:1177–1179. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

