Antiarrhythmic drug-induced internalization of the atrial-specific k+ channel kv1.5
- PMID: 19443837
- PMCID: PMC2731974
- DOI: 10.1161/CIRCRESAHA.108.192773
Antiarrhythmic drug-induced internalization of the atrial-specific k+ channel kv1.5
Abstract
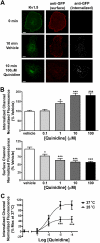
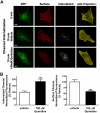
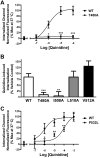
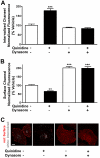
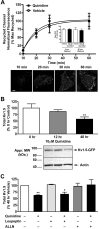
Conventional antiarrhythmic drugs target the ion permeability of channels, but increasing evidence suggests that functional ion channel density can also be modified pharmacologically. Kv1.5 mediates the ultrarapid potassium current (I(Kur)) that controls atrial action potential duration. Given the atrial-specific expression of Kv1.5 and its alterations in human atrial fibrillation, significant effort has been made to identify novel channel blockers. In this study, treatment of HL-1 atrial myocytes expressing Kv1.5-GFP with the class I antiarrhythmic agent quinidine resulted in a dose- and temperature-dependent internalization of Kv1.5, concomitant with channel block. This quinidine-induced channel internalization was confirmed in acutely dissociated neonatal myocytes. Channel internalization was subunit-dependent, activity-independent, stereospecific, and blocked by pharmacological disruption of the endocytic machinery. Pore block and channel internalization partially overlap in the structural requirements for drug binding. Surprisingly, quinidine-induced endocytosis was calcium-dependent and therefore unrecognized by previous biophysical studies focused on isolating channel-drug interactions. Importantly, whereas acute quinidine-induced internalization was reversible, chronic treatment led to channel degradation. Together, these data reveal a novel mechanism of antiarrhythmic drug action and highlight the possibility for new agents that selectively modulate the stability of channel protein in the membrane as an approach for treating cardiac arrhythmias.
Figures


References
-
- Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Bergmann JF. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane database of systematic reviews (Online) 2007:CD005049. - PubMed
-
- Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Mahe I, Bergmann JF. Antiarrhythmic drugs for maintaining sinus rhythm after cardioversion of atrial fibrillation: a systematic review of randomized controlled trials. Archives of internal medicine. 2006;166:719–728. - PubMed
-
- Nattel S, Carlsson L. Innovative approaches to anti-arrhythmic drug therapy. Nat Rev Drug Discov. 2006;5:1034–1049. - PubMed
-
- Waldo AL. A perspective on antiarrhythmic drug therapy to treat atrial fibrillation: there remains an unmet need. American heart journal. 2006;151:771–778. - PubMed
-
- Feng J, Wible B, Li GR, Wang Z, Nattel S. Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes. Circ Res. 1997;80:572–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

